Background:
Reported in the 19th century by Morvan as La chorée fibrillaire, Morvan Syndrome was classically characterized as a triad: myokymia, neuropsychiatric disturbance and insomnia. It affects more the male gender in the 5th decade of life. Myokymia (spontaneous muscular contraction) is the consequence of repetitive action potentials of peripheral nerves under hyperexcitability – Neuromyotonia or Isaac Syndrome1. If associated to dysautonomia (hyperhidrosis, cardiovascular instability, urinary retention or erectile dysfunction) and central nervous phenomena (insomnia, encephalopathy, and hallucinations) it is called Morvan Syndrome. Although the high incidence of these findings (insomnia in 90% of cases and dysautonomia up to 93%), peripheral polyneuropathy is usually the first symptom2. Typically it has a major sensitive component associated with osteotendinous areflexia and nervous hyperexcitability. It is usually associated with specific antibodies. Antibodies against contactin-associated protein-like 2 (CASPR2) are identified in the myelinated axons of the peripheral nerves and hippocampus. Association with concurrent neoplasm is very characteristic (up to 50% of Morvan Syndrome patients), mainly thymoma. Leuchine-rich glioma inactivated protein 1 antibodies (LGI1) are also identified in the hippocampus but are more associated with seizures, amnesia, hyponatremia and have a better prognosis. Although the high specificity of these antibodies it is not mandatory its presence on Morvan Syndrome3-4. On the other hand, the association of this particular tumor and autoimmune diseases (AID) is well known and in the particular case of this syndrome it has been described in 30 to 40% of cases5-6. The resolution will always require the withdrawal of the stimuli (such as thymectomy) with the potential association of immunotherapy. Classically it is administered corticosteroids, intravenous immunoglobulin or immunossupressants, but recent reports shave shown clearly that the paraneoplastic Morvan cases had worst response to conventional immunosuppression. Rituximab is a chimeric monoclonal antibody against the protein CD20, which is found primarily in the surface of B cells. Therefore this molecule is utilized to treat conditions driven by overactive or dysfunctional humoral immune defense7. The only on-label indication in AID is refractory rheumatoid arthritis, albeit the extensive use of it on many conditions, such as the neurologic syndromes refractory to conventional therapies. It has also been described its usefulness in Morvan Syndrome5,8-10.
The authors describe a case of paraneoplastic Morvan Syndrome with positivity to both anti-CASPR2 and anti-LGI1 antibodies secondary to thymoma, only responsive to rituximab (after thymectomy and conventional therapy). It will be emphasized atypical clinical findings, the role of rituximab in this refractory situation and the clinical relapse after a new stimulus emerged (a distinct neoplasm – renal cell carcinoma), once more responsive to the anti-CD20 molecule. This case report is significant because it is the first case of renal cell carcinoma presenting as Morvan Syndrome.
Case presentation
A 69 year-old man with a history of arterial hypertension and sleep apnoea presented in the emergency department with dysesthesia, muscle weakness in the lower limbs and dysautonomia. The patient had been well until 4 weeks before admission when he noticed numbness and burning sensation in the feet with progression to the knees, later associated with urinary retention, erectile dysfunction, diarrhoea and hyperhidrosis and insomnia. Before admission, muscle weakness in the legs emerged.
On the emergency department the physical examination was normal except for mild and symmetrical paraparesis with fasciculation of all limbs, tongue and chest. Blood analysis showed 11.200 white blood cells per cubic millimeter, with 69% of neutrophils. No other alterations were detected. Cerebral and dorsal spine magnetic resonance imaging (MRI) had no expansive lesions. Lumbar puncture excluded infection, neoplasm or the presence of autoantibodies. Electromyography of the upper and lower limbs revealed loss of motor units associated with multiple and spontaneous electrical discharges, compatible with myokymia. No alterations of the fibers, neurotransmission or neuromuscular junction were detected. An extensive search for autoantibodies was performed, with identification of Anti-CASPR2 and Anti-LGI1 on serum. These findings raised the possibility of Morvan Syndrome and due to its association with neoplasm a chest computed tomography (CT) was performed, identifying an anterior mediastinical mass (figure 1) that was later confirmed to be a thymoma by transthoracic biopsy.
The initial treatment was benzodiazepines, oral potassium, magnesium sulfate, Intravenous immunoglobulin(IV Ig) – 0,4mg/kg/day for 5 days – later completed with thymectomy. Pathological examination of the specimen confirmed a B2 Stage Thymoma with free margins. Persistence of myokymia and the initiation of delirium (not attributable to other causes) led to plasmapheresis.
Neurologic recovery was initially observed but one month later the patient presented in the outpatient clinic with new clinical worsening – altered mental status with nocturnal delirium, insomnia, generalized myokymia and loss of the ability to walk. It was also identified, for the first time, atrial fibrillation. Once more was admitted on the clinical ward, where he initiated corticosteroids, plasmapheresis (4 sessions) and cyclophosphamide (0.5g every two weeks during 6 sessions). Due to refractory disease rituximab was attempted (375mg/m2 weekly, initially 4 weeks, completed with two more sessions two weeks after finishing the first treatment cycle). After the anti-CD20 therapy, B cell depletion and clinical remission were achieved. Anti-CASPR2 remained positive but Anti-LGI1 antibodies turned negative. Maintenance therapy with azathioprine was attempted for 6 months, interrupted after multiple infections.
One year after the first admission and diagnosis, clinical relapse occurred, with dysesthesia and hyperhidrosis. It had been 8 months after the last rituximab session and there had been repopulation of B cells. Anti-CASPR2 remained positive and total body CT showed a mass on the left kidney (1.8cm), compatible with renal cell carcinoma (figure 2). No other lesions were identified, namely on the mediastinum. Once more total clinical remission was achieved with the association of surgery (nephrectomy that confirmed the diagnosis) and immunomodulation (corticosteroids and rituximab – 3 sessions of 1g each).
Four years after the initial diagnosis, the patient remains asymptomatic without steroids, with no new lesions albeit positivity to Anti-CASPR2 stills remains.
Discussion
This clinical case is not innovative, but it is an original and unique description of the Morvan Syndrome. In a same patient clinical relapse occurred with distinct neoplastic stimuli, both responding to rituximab. Besides that, it is the first report of a case of renal cell carcinoma presenting as Morvan Syndrome. It also stands out its clinical and serologic particularities, some never before described. Like the published literature, the first manifestation of disease involved the peripheral nerves and similar to the majority of patients, dysautonomia was also observed. Usually the voltage-gated potassium channel antibodies have a relation with the pathophysiology of the disease and are not both positive in the same patient. A Chinese paper described this rare fact and this clinical case has also the same feature11. Maintenance of positivity to Anti-CASPR2 after the resolution of the diseases justifies its continuous follow-up in the outpatient clinic, since its antigenic source is not well understood.
It can be hypothesized that these autoantibodies have proarrhythmic potential. It has been described the association of genetic defects on voltage-gated potassium channels and the occurrence of long QT syndrome and other arrhythmias. In fact there are cases of seropositive Morvan Syndrome with sinus tachycardia and extrasystoles, solved with plasma exchange12. Atrial fibrillation is probably explained by this mechanism.
Similar to other cases, paraneoplastic disease had clinical refractoriness to conventional and well established therapy (corticosteroids, IV Ig and plasmapheresis). Prognosis and clinical outcome was substantially modified with Rituximab9,11. This therapy has been described as a good alternative. It is a chimeric monoclonal antibody that reacts with CD20 antigen. This transmembrane protein is expressed on pre-B and mature B lymphocytes and this justifies its primary use on B cell lymphoma. Plasma blasts and stimulated plasma cells may also express this antigen and in the presence of abnormal stimuli it leads to its differentiation and antibody production. This is the biologic principle behind autoimmune diseases with humoral mechanisms and benefit of rituximab. In fact, Morvan Syndrome has also been linked to other AI diseases, namely neurologic ones like Myasthenia Gravis13. Reports of clinical improvement of refractory conditions with rituximab have also been published recently14. A recent observational study described its long term efficacy, even in late disease stages, on some patients with LGI1 antibody-associated encephalopathy15. The same mechanism probably occurs in paraneoplastic Morvan Syndrome, like the one described here. In this particular case a recurrent (but distinct) neoplasm led to an autoimmune syndrome that improved only under rituximab after abolition of the primary diseases. This outcome is maintained until the current date. This is the first case described to date of Morvan Syndrome as presentation of renal cell carcinoma. Only one case of renal cell carcinoma associated with Morvan syndrome has been described in the literature but in which the tumor was initially diagnosed and only later did the syndrome arise16 and a case where the presentation of a renal cell carcinoma was a limbic encephalitis17.
Conclusion
Morvan Syndrome is a rare entity with few cases described and without systematic reviews in the literature. The interest in this clinical case is the association of its prototype features, such as the neurologic symptoms, presence of voltage-gated potassium channel antibodies and association with thymoma and renal cell carcinoma. This set of findings should be well investigated by the clinician in the suspicion or presence of this disease, namely the presence of a new and pathophysiologically distinct neoplasm. Rituximab has an enormous potential as a therapeutic weapon for refractory disease and investigations regarding its mechanism of action in these subsets of diseases should be subject of future research.
Conflict of interest
The authors declare they have no conflicts of interest.
Compliance with ethical standards
All persons gave their informed consent prior to their inclusion in the study. Details that might disclose the identity of the subjects under study have been omitted. Written informed consent was obtained from the patient for publication of this Case report and any accompanying data.
Figura I
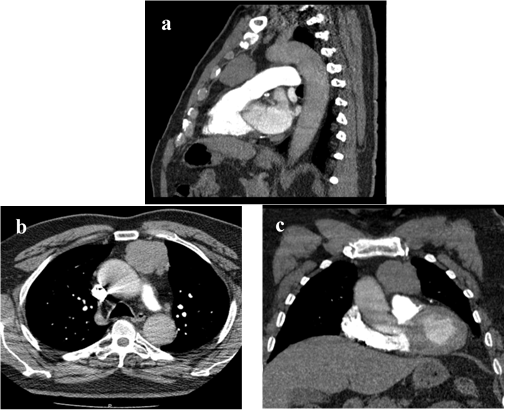
Chest CT scan with intravenous contrast administration. Sagittal (a), axial (b) and coronal (c) sections, showing an anterior mediastinal lobulated mass, 4.4x5.4x4.9cm, suggestive of thymoma. There is no invasion of adjacent structures.
Figura II
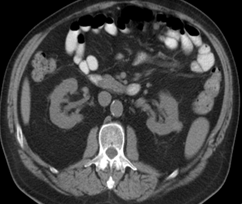
Abdominal CT scan (axial section) with intravenous contrast administration shows a small exophytic lesion (1,8cm) in the upper third of the left kidney suggestive of renal cell carcinoma.
BIBLIOGRAFIA
1. Arimura K, Watanabe O (2010) Immune-mediated neuromyotonia (Isaac’s Syndrome) – Clinical aspects and pathomecanism. Brain Nerve 62 (4): 401-10.
2. Serratrice G, Azulay JP, Serratrice J, Attarian S (2004) From Morvan’s disease to potassium channelopathies. Bull Acad Natl Med 188(2): 233-44.
3. Abou-Zeid E, Boursoulian LJ, Metzer WS, Gundogdu B (2012) Morvan syndrome: a case report and review of the literature. J Clin Neuromuscul Dis 13 (4): 214-27.
4. Irani SR, Pettingill P, Kleopa KA, Schiza N, Waters P, Mazia C, et al (2012) Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol 72 (2): 241-55.
5. Bernard C, Frih H, Pasquet F, Kerever S, Jamilloux Y, Tronc F, et al (2016) Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 15 (1): 82-92.
6. Macaron G, Rassy EE, Koussa S (2016) Morvan syndrome secondary to thymic carcinoma in a patient with systemic lupus erythematosus. Case Rep Neurol Med 2016: 9142486.
7. Gürcan HM, Keskin DB, Stern JN, Nitzberg MA, Shekhani H, Ahmed AR (2009) A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol 9(1): 10-25.
8. Ong E, Viaccoz A, Ducray F, Pérol M, Cavillon G, Rogemond V, et al (2013) Dramatic improvement after rituximab in a patient with paraneoplastic treatment-refractory Morvan syndrome associated with anti-CASPR2 antibodies. Eur J Neurol 20: e96-7.
9. Maskery M, Chhetri SK, Dayanandan R, Gall C, Emsley HCA (2016) Morvan syndrome: a case report with patient narrative and video. Neurohospitalist 6 (I): 32-5.
10. Díaz-Manera J, Rojas-Garcia R, Gallardo E, Juaréz C, Martínez-Domeño A, Martínez-Ramírez S, et al (2007) Antibodies to AChR, MuSK and VGKC in a patient with myasthenia gravis and Morvan’s syndrome. Nat Clin Pract Neurol 3 (7): 405-10.
11. Zhang L, Lu Q, Guan H-Z, Mei J-H, Ren H-T, Liu M-S, et al (2016) A chinese female Morvan patient with LGI1 and CASPR2 antibodies: a case report. BMC Neurology 16: 37.
12. Liguori R, Vincent A, Clover L, Avoni P, Plazzi G, Cortelli P, et al (2001) Morvan’s syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain 124: 2417-26.
13. Weiss M (2012) Rituximab therapy for Morvan syndrome associated with myasthenia gravis. Muscle Nerve 46 (1): 139-40.
14. Sadnicka A, Reilly MM, Mummery C, Brandner S, Hirsch N, Lunn MP (2011) Rituximab in the treatment of three coexistent neurological autoimmune diseases: chronic inflammatory demyelinating polyradiculoneuropathy, Morvan syndrome and myasthenia gravis. J Neurol Neurosurg Psychiatry 82 (2): 230-2.
15. Irani SR, Gelfand JM, Bettcher BM, Sinqhal NS, Geschwind MD (2014) Effect of Rituximab in Patients With Leucine-Rich, Glioma Inactivated 1 Antibody-Associated Encephalopathy. JAMA Neurol 71(7): 896-900.
16.Edeghere S, Browne D, McLean B (2016) Morvan’s syndrome: could insulin like growth factor-1 be a marker? Endocrine Abstr 44: 150.
17. Harrison JW, Cherukuri R, Buchan D (2015) Renal cell carcinoma presenting with paraneoplastic hallucinations and cognitive decline from limbic encephalitis. J Gen Intern Med 30 (7): 1037-40.



