Introduction
Takayasu arteritis (TA) is a chronic vasculitis of uncertain aetiology that mainly affects the aorta and its main branches and is characterized by the development of stenotic lesions. It is more common in women (80-90% of cases), the age of onset is typically between 10 and 40 years and, even though it can occur in individuals of all races and geographic regions, its incidence is higher in Asia.1 It has a wide range of manifestations, depending on the severity of the injuries and on the affected vascular beds.
Crohn’s disease (CD) is a chronic inflammatory disease that affects the gastrointestinal tract. It has a slight female preponderance, is more common among Caucasians and the symptoms onset usually occurs between the 2nd and 3rd decades. The ileum and colon are the most commonly involved sites, and symptoms like diarrhea, abdominal pain and weight loss are typical. However, systemic complications and extra-intestinal manifestations are frequent.2
Both TA and CD are inflammatory granulomatous processes but with different target organs. Even though the probability of both diseases occurring, by chance alone, in the same individual is as low as 1/5x109, 3 there are about 40 cases reported in world literature, which suggests that this association is more than a coincidence.
Case report
A 34-year-old Caucasian female was admitted to our Autoimmune Diseases Outpatient Clinic in 2006, due to total occlusion of both common carotid arteries.
Her past medical history included a viral myocarditis when she was 20 years old, and she had a eutocic delivery after a full-term uncomplicated pregnancy at the age of 27. The only data regarding the cardiac pathology that we’ve had access to are from the time of this birth – reference is made to a viral myocarditis that evolved to dilated cardiomyopathy, with the echocardiogram showing mild compromise of the left ventricular systolic function and mild mitral regurgitation. She was medicated with ramipril 5mg/d and clinically was in New York Heart Association functional class II.
At the age of 32, after a hospitalization because of abdominal pain and diarrhea, she was diagnosed with CD and was discharged on mesalazine 3g/d. Two months later, on a follow-up visit, prednisolone 20mg/d was added due to absent control of disease activity (Crohn's Disease Activity Index – CDAI=236) and evidence of a small bowel fistula. She was re-admitted 7 months later with several abscesses adjacent to the last ileal loop, and was submitted to an ileo-ceco-colonic resection. The histological examination of the surgical piece was consistent with the diagnosis of CD. In the months that followed the surgical procedure she remained clinically stable (CDAI=28), medicated with mesalazine 3g/d, and no complications were listed. Two years after the diagnosis of CD, in a routine visit, and being asymptomatic, a systolic bruit was identified over her right cervical area. The doppler ultrasound revealed total occlusion of both common carotid arteries and a normal calibre right vertebral artery with an accentuated elevation of its systolic peak velocity, of compensatory nature, and related to the audible bruit. She was therefore sent to our Clinic and after a thorough clinical questionnaire it was possible to identify the onset of left arm claudication more than 10 years earlier, which she disregarded, and occasional episodes of dizziness associated with prolonged periods of head extension. At physical examination, we noticed the absence of left brachial pulse and a difference of more than 30 mmHg in systolic blood pressure between arms (left: 101/48 mmHg; right: 133/83 mmHg). A pan-aortography was performed showing: 1) total occlusion of both common carotid arteries; 2) occlusion of the left subclavian artery at its origin; 3) concentric stenosis (of 50%) of the brachiocephalic trunk; 4) overdeveloped right vertebral artery, being the only source of brain irrigation; 5) no significant injuries in the thoracic aorta; 6) occlusion of the superior mesenteric artery; 7) occlusion of the right renal artery. Echocardiogram revealed normal-sized cardiac cavities with preserved global systolic function and mild mitral insufficiency, and cardiac catheterization showed no evidence of coronary injuries. Laboratory tests were unremarkable: erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) within normal range; normal renal function and urinalysis; negative autoimmunity (ANA, ANCA, SSA, SSB, RNP, Sm, dsDNA, anticardiolipin, lupic anticoagulant). We established the diagnosis of TA, according to the American College of Rheumatology 1990 classification criteria (table 1), with angiographic distribution type V (figure 1), and started her on prednisolone 1 mg/Kg/d (subsequently reduced to 10 mg on alternate days), methotrexate 10 mg/week and acetylsalicylic acid 100 mg/d.
In the 5 years that followed the patient remained asymptomatic, in clinical and colonoscopic remission, with no evidence of ischemic events and the routine magnetic resonance angiographies did not reveal vascular injuries apart from those described initially, nor additional thickenings of the arterial walls suggestive of active vasculitic process. However, in October 2011 she started complaining of inflammatory back pain and bilateral pain in the buttocks with morning stiffness of about 60 minutes. Schober test was positive (limitation of lumbar flexion) and so was Patrick sign bilaterally. Laboratory tests showed an increase in acute-phase proteins (ESR=24 mm, CRP=12.0 mg/dL) and MRI revealed bilateral sacroiliitis. In the colonoscopy there was no evidence of CD relapse. Facing up this new finding, we chose to associate adalimumab 40 mg every 2 weeks to methotrexate, which resulted in the control of articular complaints. To this day, we did not ascertain any complication correlated to anti-TNFα therapy and she remains in remission both from TA and CD (CDAI=16).
Discussion
The coexistence of TA and CD is rare, and was described for the first time in 1976.4 Since then, it has been reported increasingly. In a simplistic manner, one could assume this association merely reflects two diseases typically presenting in the same gender and age group. However, doubt remains about the possibility of it being the result of common pathophysiological mechanisms. In fact, TA and CD share several characteristics: female predominance with typical age of onset between the 2nd and 3rd decades of life; identical distribution of vascular injuries5, with the development of granulomatous lesions;6 the role of cell-mediated immunity in their pathophysiology and the inflammatory process behind both diseases, which is largely influenced by pro-inflammatory cytokines, namely the tumor necrosis factor alpha (TNFα), and interleukins (IL) IL-6, IL-8, IL-12 and IL-18.7 Supporting this theory, there are several studies that attest that CD prevalence among patients with TA is significantly higher than that described in general population: Hall, et al 8 reported 2 cases of CD in a population of 32 north-American patients with TA; 2) Kerr et al. 9 followed-up 64 patients with TA, of which 2 had CD and 2 had ulcerative colitis; 3) Reny et al. 5 retrospectively studied a series of 44 patients with TA and noticed that 9% had CD. Whilst these evidences, until this day no definite etiological association was established and common HLA haplotypes were not identified.7
In 88% of the cases reported in literature, the diagnosis of TA is established simultaneously or after the presentation of CD.10 In our patient, the diagnosis of CD also preceded that of TA. However, the clinical data suggest that TA was diagnosed in a quiescent phase, which is not uncommon, and that its onset occurred several years before, when arm claudication begun. The fact that no major ischemic events occurred in spite of the extent of the angiographic findings also supports the idea that TA had a long course. One might also feel tempted to presume that the viral myocarditis at age 20 was mislabelled, and that it was in fact the first manifestation of TA. Nevertheless, the lack of clinical data does not allow us to make that assumption.
As it is clear from the case reported, it can be extremely difficult to recognise TA. It is important that clinicians who follow-up CD patients (chiefly females under 40) bear in mind the association with TA, being systematic in the assessment of vascular signs and symptoms, so that more cases are identified and in earlier stages. Our patient, even if asymptomatic, had several signs (the bruit, the pulselessness, the difference of systolic arterial pressure between arms) which were overlooked, possibly for several years.
The scarce amount of cases of TA associated with CD, and consequent absence of randomized controlled studies, make it difficult to reach a consensus about its management. From reviewing the reported cases in literature, it is clear that the experience of each centre in treating TA or CD alone often prevails. In the case here reported, immunosuppression with methotrexate was effective in maintaining both diseases in remission for 5 years. However, the diagnosis of enteropathic spondyloarthropathy forced us to consider other options, namely the association of a biologic drug. The strategy was to use a drug with proven efficacy in CD and associated arthritis but effective in treating TA as well. Among TNFα-inhibitors both infliximab and adalimumab have been successfully used in refractory cases of CD and TA.11,12 Adalimumab was chosen once the patient manifested her preference for a subcutaneous instead of an intravenous drug, and until now the results have been encouraging, with control of articular complaints and remission of TA and CD.
Conclusion
Facing a patient with TA or CD, it is essential to bear in mind the association of both diseases, so that all the signs and symptoms are properly assessed and diagnosis is promptly established.
Regarding the treatment of this association, biologic drugs are promising although randomized trials are required to define the best therapeutic approach. Nevertheless, current investigation to ascertain the pathophysiological mechanisms underlying TA and CD should be encouraged, in the pursuit of new therapeutic strategies.
Quadro I
Criteria for the classification of TA, American College of Rheumatology 1990
| Criteria | Definition |
| | |
| Age at disease onset < 40 years | Development of symptoms or findings related to TA at age ≤ 40 |
| Claudication of extremities | Development and worsening of fatigue and discomfort in muscles of 1 or more extremity while in use, especially the upper extremities |
| Decreased brachial artery pulse | Decreased pulsation of 1 or both brachial arteries |
| Blood pressure difference >10 mmHg | Difference of >10 mmHg in systolic blood pressure between arms |
| Bruit over subclavian arteries or aorta | Bruit audible on auscultation over 1 or both subclavian arteries or abdominal aorta |
| Arteriogram abnormality | Arteriographic narrowing or occlusion of the entire aorta, its primary branches, or large arteries in the proximal upper or lower extremities, not due to arteriosclerosis, fibromuscular dysplasia, or similar causes; changes usually focal or segmental |
* For purposes of classification, a patient shall be said to have TA if at least 3 of these 6 criteria are present.
Figura I
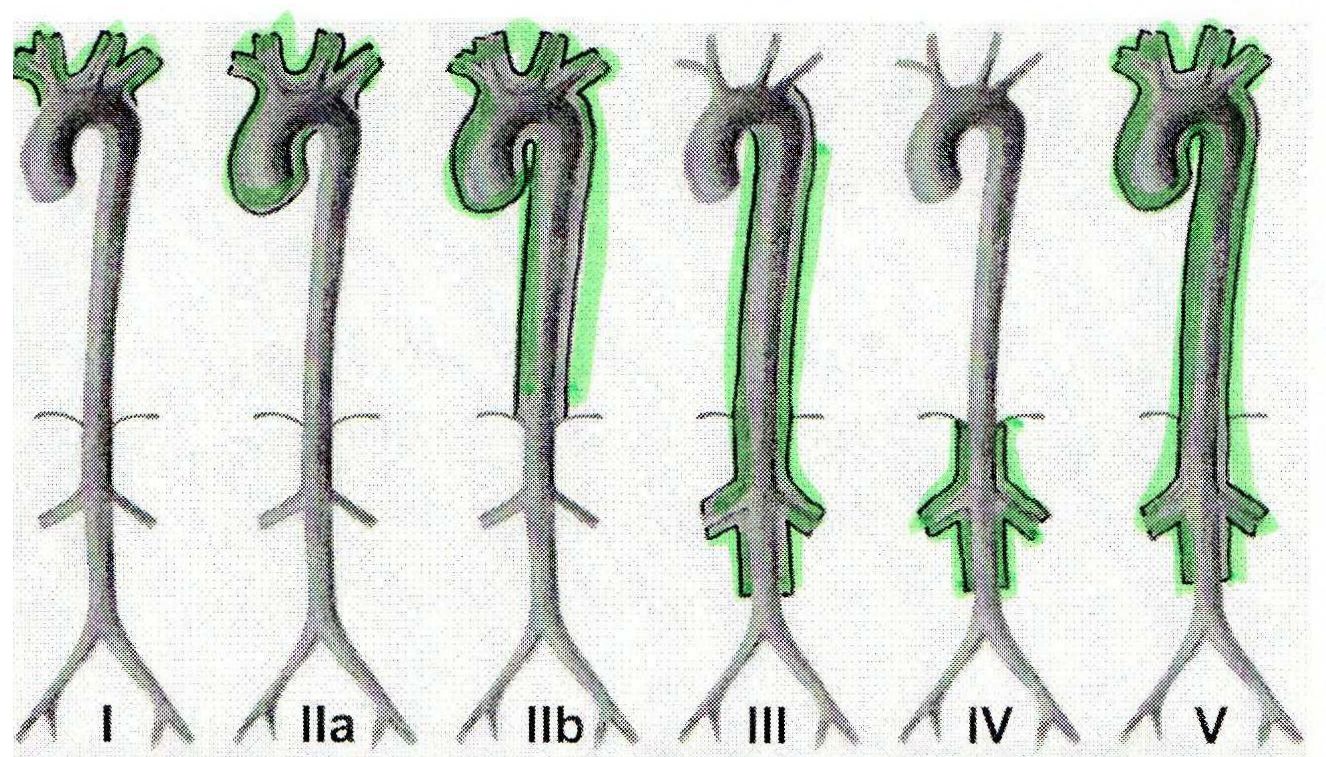
Angiographic classification of TA, Takayasu conference 1994
BIBLIOGRAFIA
1. Weyand CM, Goronzy JJ. Vasculitides. In: Klippel JH, Stone JH, Crofford LJ, White PH, editors. Primer on the Rheumatic Diseases. 13th ed. New York: Springer 2008; p.398-450.
2. Karlinger K, Gyorke T, Mako E, Mester A, Tarjan Z. The epidemiology and the pathogenesis of inflammatory bowel disease. Eur J Radiol 2000; 35:154-67.
3. Lenhoff SJ, Mee AS. Crohn’s disease of the colon with Takayasu’s arteritis. Postgrad Med J 1982;58:386-9.
4. Yassinger S, Adleman R, Cantor D, Halsted CH, Bolt RJ. Association of inflammatory bowel disease and large vascular lesions. Gastroenterology 1976;71:844-6.
5. Reny JL, Paul JF, Lefèbvre C, Champion K, Emmerich J, Blétry O, et al. Association of Takayasu’s arteritis and Crohn’s disease. Results of a study on 44 Takayasu patients and review of the literature. Ann Med Interne (Paris) 2003;154(2):85-90.
6. Wakefield AJ, Sankey EA, Dhillon AP, Sawyerr AM, More L, Sim R, et al. Granulomatous vasculitis in Crohn’s disease. Gastroenterology 1991;100: 1279-87.
7. Ratuapli S, Mazlumzadeh M, Gurudu S, Money S, Heigh R. Coexisting Crohn’s Disease and Takayasu’s Arteritis in Two Patients Treated with Anti-TNFα Therapies. Case Rep Gastroenterol 2010;4:35-40.
8. Hall S, Ball W, Lie JT, Stanson AW, Kazmier FJ, Hunder GG. Takayasu’s arteritis: a study of 32 North American patients. Medicine (Baltimore) 1985; 64(2):89-99.
9. Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, Hoffman GS. Takayasu arteritis. Ann Intern Med 1994;120(11):919-29.
10. Farrant M, Mason JC, Wong N, Longman RJ. Takayasu’s arteritis following Crohn’s disease in a young woman: Any evidence for a common pathogenesis? World J Gastroenterol 2008;14(25):4087-90.
11. Molloy ES, Langford CA, Clark TM, Gota CE, Hoffman GS. Anti-tumour necrosis factor therapy in patients with refractory Takayasu arteritis: long-term follow-up. Ann Rheum Dis 2008;67(11):1567-9.
12. Tatò F, Rieger J, HoffmannU. Refractory Takayasu’s arteritis successfully treated with the human monoclonal anti-tumor necrosis factor antibody adalimumab. Int Angiol 2005;24(3):304-7.


