BACKGROUND
Olmesartan is an anti-hypertensive drug in use since 2002. The association between olmesartan and a sprue-like enteropathy was first described by Rubio-Tapia et al. in August 2012. The clinical features of this enteropathy are: chronic diarrhoea with weight loss; negative celiac serology tests; evidence of sprue-like enteropathy (villous atrophy) with or without collagen deposition or intraepithelial lymphocytosis; lack of clinical response to gluten-free diet; exclusion of other causes of enteropathy; evidence of clinical and histologic improvement after suspension of olmesartan.[1]
CASE PRESENTATION
In December 2012, a hypertensive and dyslipidaemic 65-year-old woman with hepatic steatosis, chronically medicated with olmesartan 20 mg, amlodipine 5 mg, indapamide 2,5 mg and sinvastatine 20 mg, presented with watery diarrhoea without blood or mucous, abdominal pain and early post-prandial vomiting, for 3 weeks, causing weight loss of 5 kg since the beginning of symptoms, and acute renal failure with hypokalaemia. She denied recent travels, sick contacts, and recent changes in diet or medication.
The patient was hospitalized and hydration and empiric antibiotherapy with cotrimoxazol was instituted, with presumed diagnosis of an infective gastroenteritis. She presented favourable outcome, although coprocultures resulted negative.
The patient was re-hospitalized in January 2013, due to recurrence of the symptoms. An extensive investigation was preformed revealing the following results:
- Normal thyroid function tests.
- Negative HIV serology.
- Negative Clostridium difficile toxin assays; no ova, cyst and parasites on stool.
- Negative IgA endomysial, IgA tissue transglutaminase, IgA and IgG gliadine antibodies.
- The upper gastro-intestinal endoscopy (Figure 1) showed haemorrhagic gastropathy, an antral polypus and a duodenal ulcer. The gastric mucosa biopsy revealed various high activity chronic gastritis lesions, and reactive faveolar hyperplasia (Figure 2). Negative to Helicobacter pylori.
- Normal colonoscopy.
- No lesions on the abdominal CT with gastrographine.
- Upper gastro-intestinal endoscopy with DII segment biopsies (Figure 3) revealed moderate chronic duodenitis with morphologic aspects compatible with celiac disease/celiac-like enteropathy.
- Negative therapeutic prove with colestiramine.
- Negative gastrinemia assay.
The patient gradually recovered with hydration, and a gluten-free diet.
Ten days after discharge the patient was again hospitalized due to recurrence of symptoms (watery diarrhoea, vomiting), dehydration, and acute renal failure with severe metabolic acidosis. The patient recovered with intensive hydration and empiric antibiotherapy with metronidazole. Additional clinical investigation consisted of a second colonoscopy which revealed mild chronic colitis (Figure 4).
The patient recovered normal renal function, presenting significant improvement of symptoms, maintaining semi-liquid dejections in lower frequency. Olmesartan associated sprue-like enteropathy was suspected so, on discharge, the anti-hypertensive drug was changed to an angiotensin converting enzyme inhibitor. The patient was recommended to maintain a gluten-free diet.
At the 1-month follow-up evaluation, the patient reported complete recovery of symptoms. On the 7-month follow-up, the patient remained asymptomatic, had gained weight and was not complying with the diet. The duodenal biopsies showed recovery of normal villous architecture (Figure 5).
DISCUSSION
The pathological mechanism underlying the recently reported association between anti-hypertensive treatment with olmesartan and a sprue-like enteropathy remains unknown.Rubio-Tapia et al. [1] proposed a mechanism related to cell-mediated immunity damage, associated to the angiotensin receptor blockers inhibitory effects on the transforming growth factor β action, an important mediator of gut immune homeostasis. Another mechanism proposed [2] relates the villous atrophy in the sprue-like enteropathy to a pro-apoptotic effect of angiotensin II on intestinal epithelial cells, mediated through AT2 receptor activation. It has also been suggested that the presence of HLA DQ2 or DQ8 may act as potential predisposing factors [1,4].Nevertheless, the clinical findings prove clinical and histologic improvements after suspension of the drug. [1, 2] Iatrogenic sprue-like enteropathy had been previously reported with mycophenolate, azathioprine, methotreaxate and interferon-alpha. [3]
A recently published systematic review [2] included 54 patients, all were negative for celiac serology tests, 98% patients presented variable degrees of duodenal villous atrophy, but only 65% had increased intra-epithelial lymphocytes. In all cases, the complete recovery of symptoms was achieved after discontinuation of olmesartan.
The first 22-case series reporting this association [1], also mentioned pathologic evidence of involvement of other organs, such as collagenous or lymphocytic gastritis and collagenous or lymphocytic colitis. Greywoode et al. investigated this association in a population of 2088 patients who underwent esophagogastroduodenoscopy or colonoscopy examinations to determine the aetiology of diarrhoea, showing no association between the use of olmesartan and the histologic diagnosis of celiac disease or microscopic colitis. They concluded that it is likely a rare adverse effect and milder presentations are unlikely. [4]
There is a unique case report [5] of olmesartan associated severe sprue-like enteropathy and colon perforation, treated conservatively without surgical intervention and olmesartan discontinuation, with complete resolution of symptoms.
The characteristic recurrence of symptoms after discharge, with reintroduction of chronic anti-hypertensive therapy might be a clue to clinical suspicion, and it reflects the major importance of recommending patients not to resume the use of olmesartan. Such as in our case report, Theophile et al. [3] report a 5 case series in which 2 patients presented rapid recurrence of diarrhoea after reintroduction of olmesartan.
CONCLUSION
Although it is likely a rare adverse effect, spruelike enteropathy associated with olmesartan can be life threatening and clinicians should be aware of this potential association.
Figura I
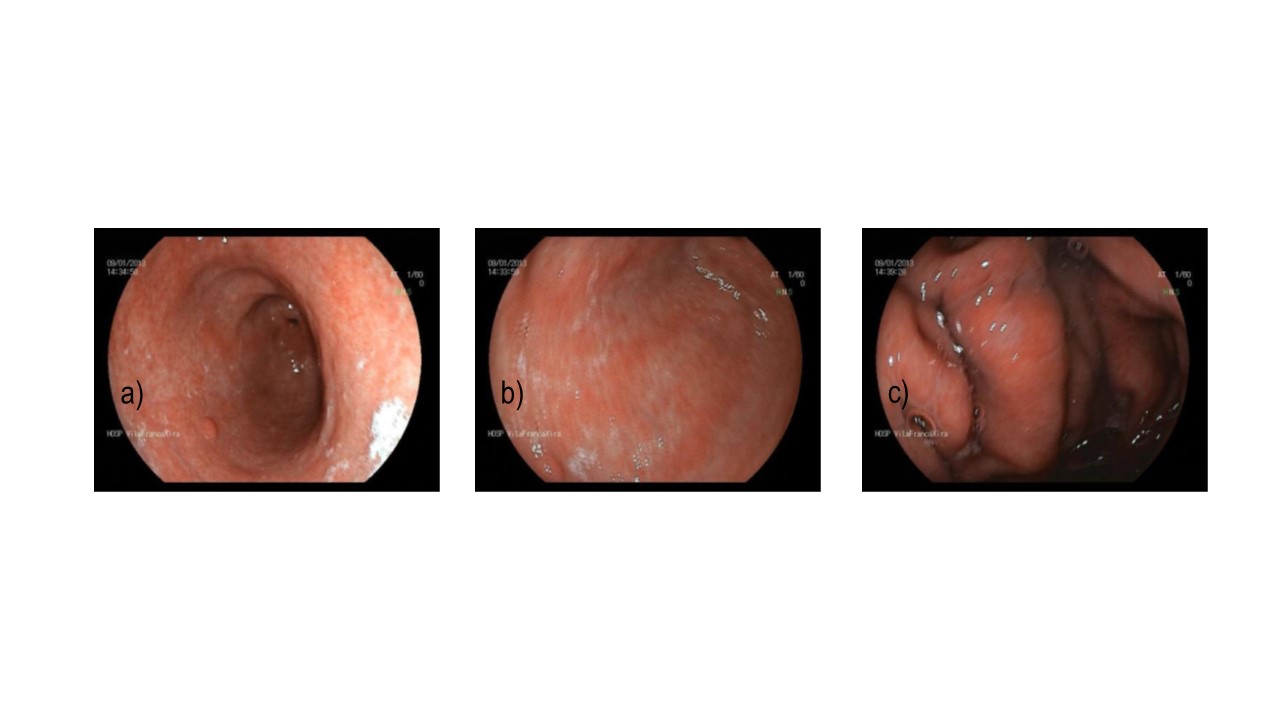
Upper gastro-intestinal endoscopy: haemorrhagic gastropathy [a) antral polypus, b) bulb, c) fundus].
Figura II
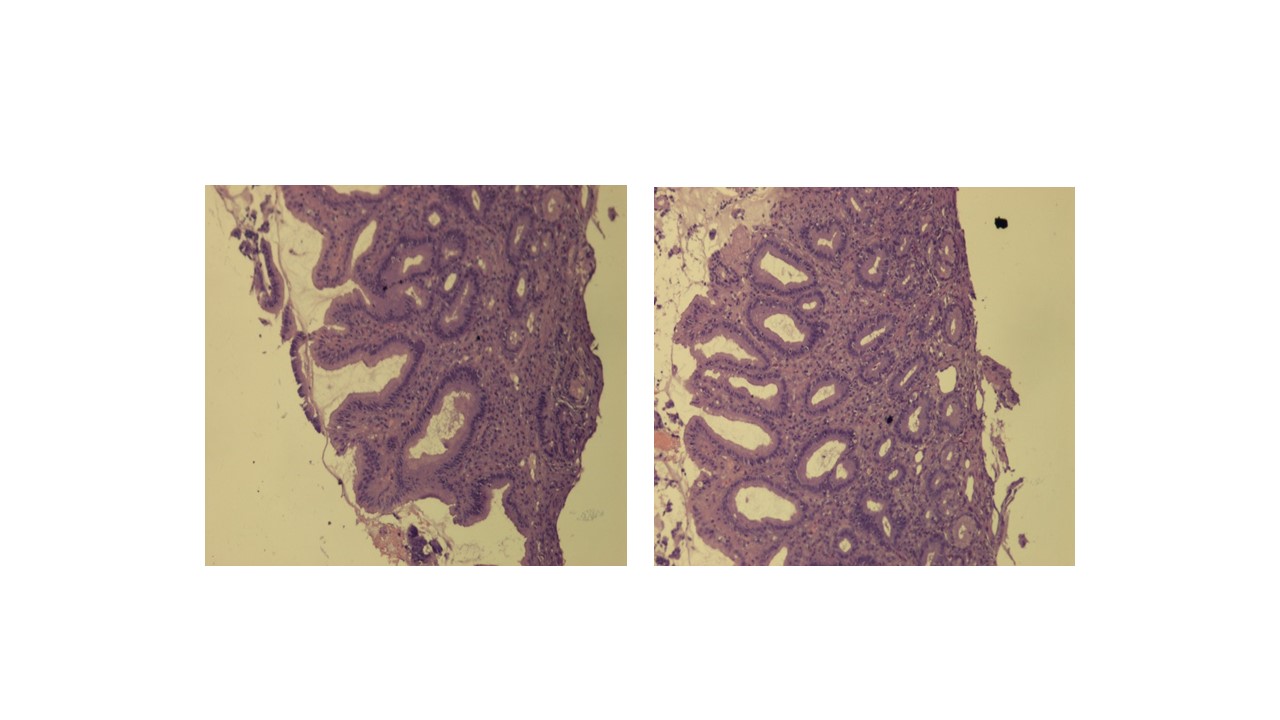
High activity chronic gastritis lesions, reactive faveolar hyperplasia (10x).
Figura III
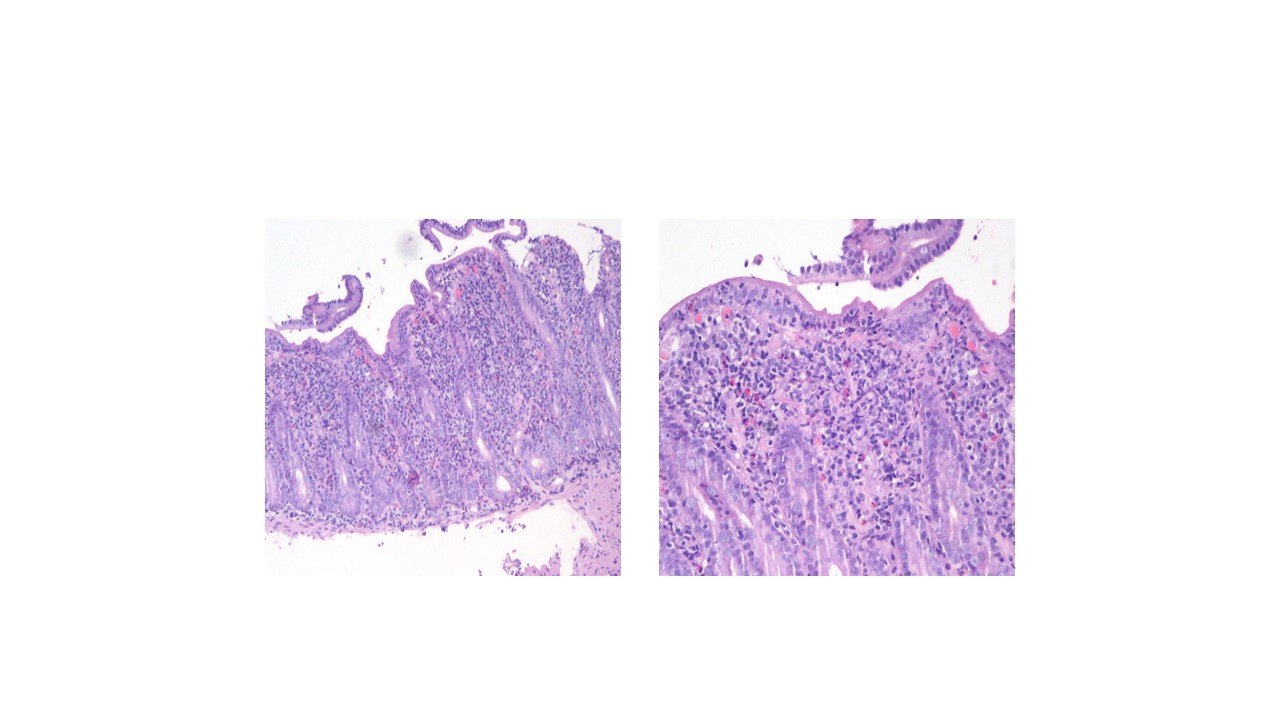
Duodenal mucosa with villous atrophy, hypertrophic crypts, intraepithelial lymphocytes and eosinophils. Moderate linfoplasmocitic inflammatory infiltrate a) 10x, b) 40x.
Figura IV
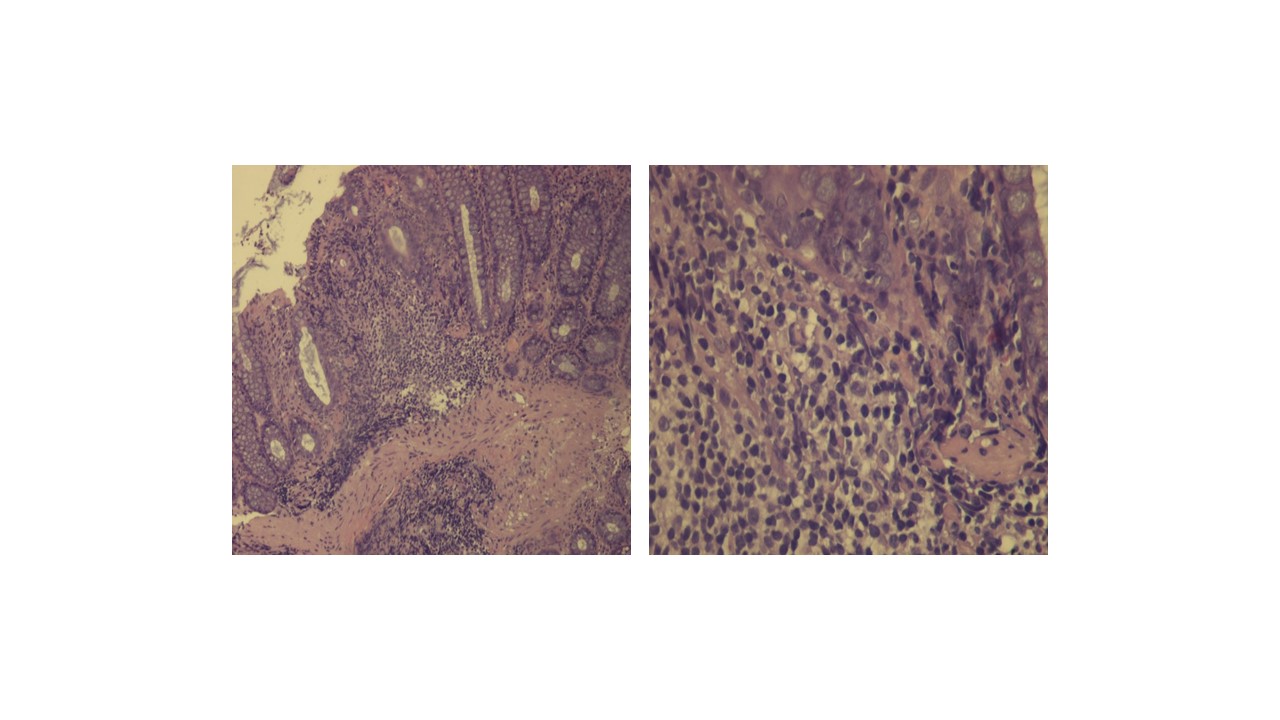
Mild chronic colitis a) 10x, b) 40x.
Figura V
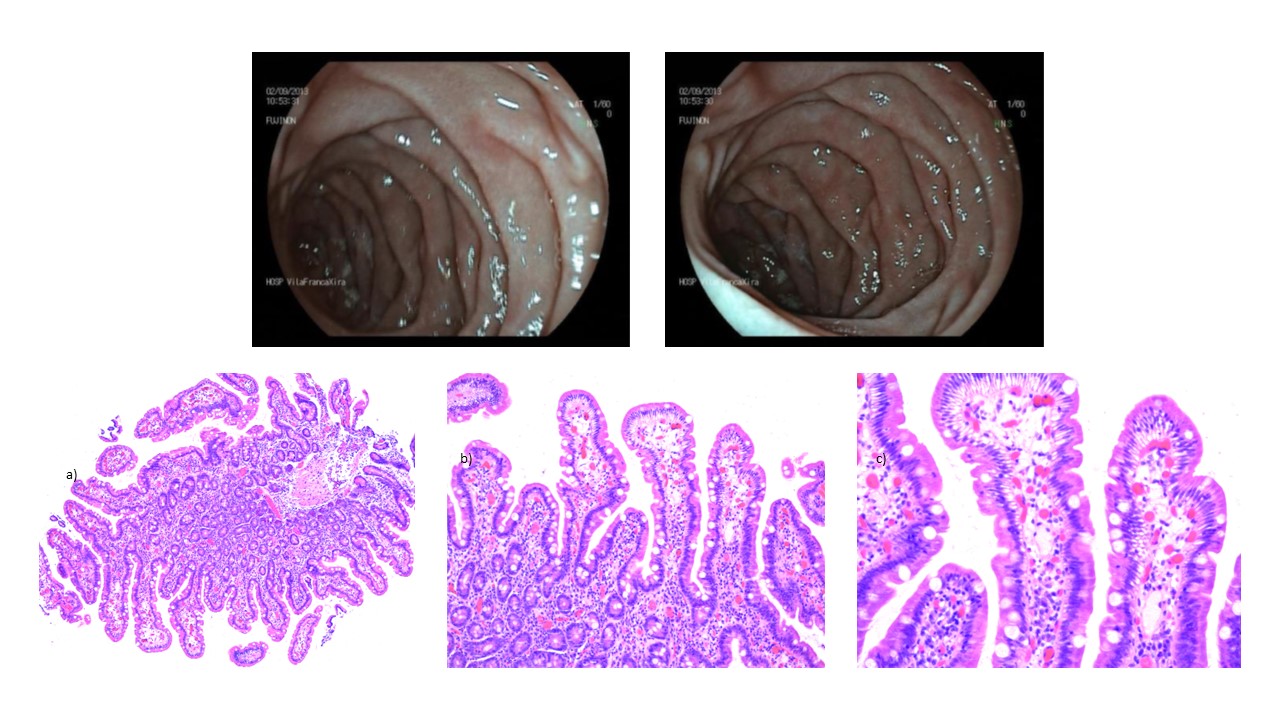
6-month follow-up upper gastro-intestinal endoscopy showing no lesions with duodenal biopsies showing recovery of normal villous architecture, a) 50x, b) 100x, c) 200x.
BIBLIOGRAFIA
[1] Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012 Aug;87(8):732-8.
[2] Ianiro G, Bibbò S, Montalto M, Ricci R, Gasbarrini A, Cammarota G. Systematic review: sprue-like enteropathy associated with olmesartan. Aliment Pharmacol Ther. 2014 Jul;40(1):16-23.
[3] Théophile H, David XR, Miremont-Salamé G, Haramburu F. Five cases of sprue-like enteropathy in patients treated by olmesartan. Dig Liver Dis. 2014 May;46(5):465-9.
[4] Greywoode R, Braunstein ED, Arguelles-Grande C, Green PH, Lebwohl B. Olmesartan, other anti-hypertensives, and chronic diarrhea among patients undergoing endoscopic procedures; a case-control study. Mayo Clin Proc. 2014 Sep;89(9):1239-43.
[5] Abdelghany M, Gonzalez L III, Slater J, Begley C. Olmesartan associated sprue-like enteropathy and colon perforation. Case Rep Gastrointest Med. 2014;2014:494098. doi: 10.1155/2014/494098.
[6] Mooney PD, Evans KE, Singh S, Sanders DS. Treatment failure in coeliac disease: a practical guide to investigation and treatment of non-responsive and refractory coeliac disease. J Gastrointestin Liver Dis. 2012 Jun;21(2):197-203.
[7] Murray JA, Rubio-Tapia A. Diarrhoea due to small bowell diaseases. Best Pract Res Clin Gastroenterol. 2012 Oct;26(5):581-600.
[8] Schiller LR. Definitions, pathophysiology and evaluation of chronic diarrhoea. Best Pract Res Clin Gastroenterol. 2012 Oct;26(5):551-62.
[9] Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc. 2014 Jan;79(1):140-50.






