INTRODUCTION
Non-Hodgkin Lymphoma (NHL) can affect the peripheral nervous system in up to 5% patients1. In the absence of direct compression or invasion, the accepted hypothesis is that a paraneoplastic neurological syndrome is caused by immune mechanisms triggered against antigens that are normally present in the peripheral nervous system and ectopically expressed by the tumour2, 3.Paraneoplastic antibodies may be absent3 and extensive investigations may be needed to find the underlying malignancy.
Chronic inflammatory demyelinating polyneuropathy (CIDP) is anacquired immune-mediated inflammatory disorder of the peripheral nervous system. Contrasting to Guilain-Barré syndrome, which has similar clinical, electromyographic and CSF findings, CIDP has a progressive or relapsing curse over 8 weeks. It is presumed to occur because of immunologic antibody-mediated reaction along with interstitial and perivascular infiltration of the endoneurium with inflammatory T cells and macrophages. The consequence is a segmental demyelination of peripheral nerves.CIDP is most commonly idiopathic, but may be associated Diabetes Mellitus, Paraproteinemias and Monoclonal Gammopathy of Unknown Origin, HIV infection, Hepatitis C Infection, Sjogren’s syndrome, Melanoma and Lymphoma. CIDP associated with hematologic malignancies characteristically involves plasma cells such as plasmacytoma or osteosclerotic myeloma4.
We report a rare case of a patient with lymphoplasmocytic lymphoma and IgM monoclonal gammapathy (also termed Waldenström macroglobulinemia),presenting as a clinically aggressive paraneoplastic CIDP, and discuss its mechanisms and treatment options.
Clinical case
A 55-year-oldCaucasian male, was admitted to the Neurology ward due to distal, symmetrical lower limb (LL) weakness. In the previous 4 weeks, he reported paraesthesia in both hands that extended proximally to the arms. Neurological examination disclosed distal paraparesis (grade 4), absent deep tendon reflexes, hypoesthesia in a “socks” distribution and abolition of vibratory and postural sensationsin distal LL.Physicalexam was otherwise unremarkable. Prior medical history included smoking and pulmonary tuberculosis. Electromyogram (EMG) performed at admission showed slight distal demyelination of the right median and left posterior tibial nerves. Cerebrospinal fluid (CSF) cytochemical and bacteriologic analysis were normal (CSF: 0,8/mm3 cells, glucose 5 mmol/l, proteins 0,39 g/l). Laboratory workup revealed normochromic normocytic anaemia (Hb 10,9 g/dL), with normal levels of iron, ferritin and total iron binding capacity. Elevated ESR (113 mm); serum glucose 9,4 mmol/l, proteins 97 g/l;IgM/K monoclonal gammapathy(IgG 24,4 g/L, IgM 13,4 g/L – 6 times the upper limit of normal, k-chains 7,05 g/L); absence of Bence Jones proteinuria; normal B2 microglobulin levels.Positive ANA (1/160) and anti-SSA antibodies.Positive Anti-HBCantibody; negative HIV serology. Positive anti-ganglioside antibodies(IgM GM3 - 1/2000, GD3 - 1/5000 and GM4 - 1/2000); negative anti-MAG, anti-Ri, anti-Hu e anti-Yo antibodies. Skeleton x-ray revealed no lytic bone lesions;normal thoracic-abdominal-pelvic CT scan.Spinal MRI (Fig. 1) showed altered signal in T1W and T2Wimages in the vertebral bodies suggesting diffuse bone marrow infiltration. The myelogram revealed a normocelular bone marrow and a myeloid/erythroid shift, with 5% lymphocytes and 1% plasmocytes. The first bone marrow biopsy (BMB) was inconclusive and he started corticotherapy 1 mg/Kg/day, without improvement. A second EMG, performed 3 weeks later, showed significantworsening of the neurophysiological picture with marked distal demyelinationin upper and lower limbsand nerve conduction blocks, fulfilling criteria for CIDP.A second CSF analysis revealed albumin-cytological dissociation (CSF: 2,4/mm3 cells, glucose 3,2 mmol/l, proteins 0,56 g/l).
Despite the administration of two IV immunoglobulin pulses, on the 16th week there was clinical progression to tetraparesis(muscle strength grade 3) and global respiratory insufficiency aggravated by pneumonia, which required UCI admission and invasive mechanical ventilation support. The third EMG revealed significant neurophysiological worsening and moderate demyelination of the phrenic nerves. A second BMB (Fig. 2) confirmed the diagnosis of lymphoplasmocytic NHL, and the patient started chemotherapy with Cyclophosphamide, Doxorubicin, Vincristine and Prednisolone (CHOP) still in the ICU, without clinical improvement. A 2nd and 3rd chemotherapy cycles with Cyclophosphamide, Doxorubicin, Etoposide (vincristine was replaced by Etoposide due to its putative neurological toxicity) and Prednisolone (R-CHEP) associated with Rituximab, were prescribed with evident neurological and neurophysiological improvement. The patient resumed daily life activities within 2 months, but not work (watchmaker). The EMG performed 1 year later was normal, while the underlying haematological condition was under remission, confirmed both clinically and by bone marrow analysis.
DISCUSSION
This case report draws attention to a rare association of an immune demyelinating polyneuropathy and lymphoplasmocytic lymphoma. Polyneuropathies are a known neurological complication of lymphoma in general.The pathophysiology includes compression or direct infiltration (neurolymphomatosis) of the nerves by neoplastic cells; metabolic, nutritional and infectious complications of lymphoma; chronic demyelination mechanisms due to paraneoplastic immune deregulation; and/or complications of treatment5. Rarer causes of neuropathy include vasculitis cryoglobulinemia, amyloidosis and ischemia due to vessel occlusion by malignant cels5.
Waldenström macroglobulinemia (WM) is a B-cell lymphoplasmocytic lymphoma characterized by the production of monoclonal immunoglobulin M protein in the serum and infiltration of bone marrow with lymphoplasmocytic cells6. In most patients the clinical curse is indolent but death can occur due to disease progression, transformation to high-grade lymphoma or complications of treatment6. Polyneuropathies are the most frequent neurological complications in WM and their incidence is about 40%7. Identification of the cause of neuropathy is important both for prognostic and therapeutic decisions. Most of the cases are due to immune mechanisms, including production of antibodies against specific neuronal antigens (including myelin associated glycoproteins (MAG) and gangliosides, endoneurial deposits of IgM and cryoglobulinemic or amyloid neuropathy caused by circulating IgM. Nerve infiltration has also been described7. Demyelinating neuropathies disclose two patterns according to the findings on anti-MAG antibodies (ab). Patients with significant titers of anti-MAG ab have a typical EMG pattern with preponderance of symmetric distal nerve demyelination and absence of conduction block (Anti-MAG neuropathy). Patients with negative anti-MAG Ab have electrophysiological features consistent with chronic idiopathic inflammatory polyneuropathy (CIDP), which has been reported to be more disabling but more responsive to immunotherapy7.
Our patient initially presented mild polyneuropathy with a predominantly distal demyelination on the EMG. The neuropathy was not disabling and had weak response to immune-modulatory treatment suggesting anti-MAG neuropathy, however these antibodies were negative. Three antiganglioside antibodies IgM-GM3,-GD3,-GM4 were identified, although these were not previously associated with a specific clinical or electrophysiological picture in the literature, in contrast with anti-GM1/GM2/GD1b8.
The patient progressed rapidly to a severe CIDP. Seven series of patients with CIDP with concomitant paraproteinaemia were reported since 1991 and the prevalence of MGUS or Waldenström disease ranged from 13 to 47%10. In a recent series (30), 12% of patients with MAG-polyneuropathy had WM at the time of diagnosis11. At a mean follow-up time of 8.5 years, 9% of patients with IgM-MGUS developed WM.
Early medical treatment directed at the tumour and the CIDP is important to prevent axonal loss. Only three treatment regimens for CIDP have demonstrated benefit in randomized, controlled studies: corticosteroids, plasma exchange, and intravenous immunoglobulins12. On the other hand large placebo-controlled trials with alternative immunosuppressive agents or monoclonal antibodies are still lacking12, but, the administration of the humanized monoclonal antibody directed against B cell lymphocytes CD20 (rituximab) is currently defended in the treatment of autoimmune manifestations of lymphoproliferative diseases that do not respond to standard chemotherapy, or even in combination with primary chemotherapy13. Multiple combination chemotherapy seems to result in better CIDP remission rates in comparison with standard isolated chemotherapy14. Rituximab, with proven efficacy in the presence of anti-MAG and anti-GM1 auto-antibodies14,eliminates circulating CD20+ B cells15, preventing the production of auto-antibodies and promoting neurophysiological and clinical recovery in cases of IgM antibody associated polyneuropathies14.The better response to rituximab includes a faster improvement onset and a shorter duration of neurological symptoms13.
In this case also, CIDP seems to have responded only after adding rituximab to the chemotherapy regimens.
CONCLUSIONS
This case of severe CIDP was associated with Waldenström´s macroglobulinemia and IgM anti-GM3,-GD3,-GM4, and it was refractory to standard therapy of CIDP. Clinical recovery started with cytostatic directed therapy, with striking improvement after rituximab was added, which further argues in favour of a specific synergistic effect of rituximab in the CIDP resolution.
ACKNOWLEDGMENTS
The authors wish to thank Drª. Cândida Barroso, MD, Neurological Pathology Department, for her cooperation in the histopathology services.
Figura I
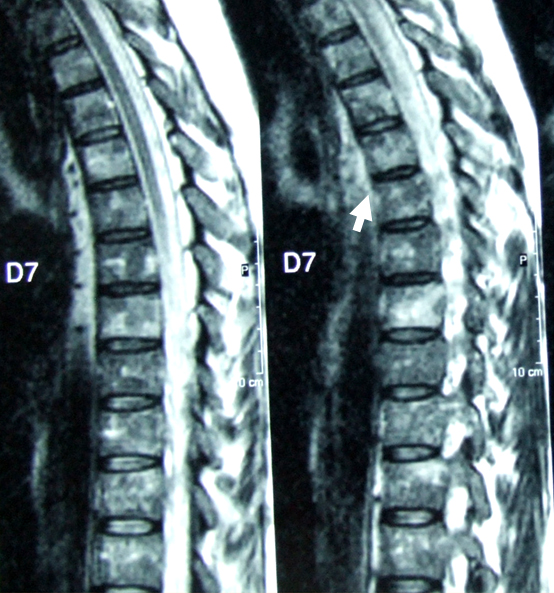
sagital dorsal MRI T2*: altered vertebral body signal suggesting difuse bone marrow infiltration
Figura II
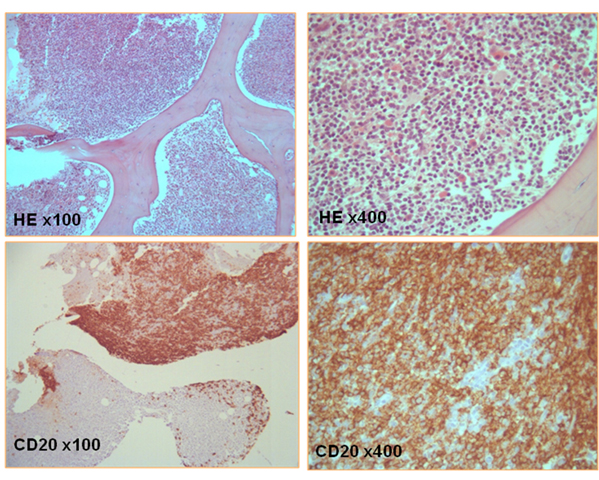
Bone marrow biopsy: small lymphoid cell infiltration with rounded chromatin nucleus, located peripherically; lymphoplasmocytic cells with amphophyle cytoplasm; Immuno-chemistry profile showing exclusive CD20 (B cells) phenotype.
BIBLIOGRAFIA
1. Hughes RA, Britton T and Richards M. Effects of lymphoma on the peripheral nervous system. J R Soc Med. 1994; 87: 526-30.
2. Kelly JJ and Karcher DS. Lymphoma and peripheral neuropathy: a clinical review. Muscle Nerve. 2005; 31: 301-13.
3. Graus F, Arino H and Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014; 123: 3230-8.
4. Kelly JJ, Jr., Kyle RA, Miles JM and Dyck PJ. Osteosclerotic myeloma and peripheral neuropathy. Neurology. 1983; 33: 202-10.
5. Antoine JC and Camdessanche JP. Peripheral nervous system involvement in patients with cancer. Lancet Neurol. 2007; 6: 75-86.
6. Oza A and Rajkumar SV. Waldenstrom macroglobulinemia: prognosis and management. Blood Cancer J. 2015; 5: e296.
7. Viala K, Stojkovic T, Doncker AV, et al. Heterogeneous spectrum of neuropathies in Waldenstrom´s macroglobulinemia: a diagnostic strategy to optimize their management. J Peripher Nerv Syst. 2012; 17: 90-101.
8. European Federation of Neurological S, Peripheral Nerve S, Hadden RD, Nobile-Orazio E, Sommer C, et al.European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of paraproteinaemic demyelinating neuropathies: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2006; 13: 809-18.
9. Cocito D, Durelli L and Isoardo G. Different clinical, electrophysiological and immunological features of CIDP associated with paraproteinaemia. Acta Neurol Scand. 2003; 108: 274-80.
10. Nobile-Orazio E, Meucci N, Baldini L, Di Troia A and Scarlato G. Long-term prognosis of neuropathy associated with anti-MAG IgM M-proteins and its relationship to immune therapies. Brain. 2000; 123 ( Pt 4): 710-7.
11. Yoon MS, Chan A and Gold R. Standard and escalating treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Ther Adv Neurol Disord. 2011; 4: 193-200.
12. Kilidireas C, Anagnostopoulos A, Karandreas N, Mouselimi L and Dimopoulos MA. Rituximab therapy in monoclonal IgM-related neuropathies. Leuk Lymphoma. 2006; 47: 859-64.
13. Pestronk A, Florence J, Miller T, Choksi R, Al-Lozi MT and Levine TD. Treatment of IgM antibody associated polyneuropathies using rituximab. J Neurol Neurosurg Psychiatry. 2003; 74: 485-9.
14. Dalakas MC, Rakocevic G, Salajegheh M, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein antibody demyelinating neuropathy. Ann Neurol. 2009; 65: 286-93.



