Introduction
Paclitaxel and trastuzumab are two antineoplastic drugs commonly used in the treatment of metastatic HER-2 positive breast cancer. Both have many side effects. Interstitial pneumonitis is a rare adverse effect of both therapies, described in less than 1% of the cases1-2.
The diagnosis of drug-induced lung toxicity is challenging and one of exclusion. Many hypothesis must be formulated, especially considering the unspecific presentation of this pathology and the immunosuppressive state of the patients3. A rapid diagnosis can prevent further morbidity and even, in severe cases, mortality.
The authors report a case of lung toxicity after use of paclitaxel and trastuzumab in a metastatic breast cancer patient.
Clinical Case
A 71-year-old woman was admitted in the emergency service, in October 2016, complaining of fatigue and dry cough.
She had history of mellitus diabetes type 2 treated with oral antidiabetics and breast cancer.
The breast cancer was diagnosed 4 years earlier, in January 2012. She presented with a locally advanced lesion staged as cT3N+M0. Immunohistochemical evaluation showed HER2 overexpression (3+), estrogen receptor positive (90%) and progesterone receptor negative. She started neoadjuvant chemotherapy (ChT) with FEC-DH regimen (4 cycles of 5-fluorouracil (500mg/m2), epirubicin (100mg/m2), cyclophosphamide (600mg/m2) q21 days, followed by 4 cycles of docetaxel (75mg/m2) and trastuzumab (6mg/Kg). In August 2012, she performed a modified radical left mastectomy. Pathology evaluation presented ypT1cN3aG3R0. After surgery, she completed 1 year of trastuzumab alongside with adjuvant hormone therapy with anastrazol and adjuvant thoracic radiotherapy (RT) of regional lymph nodes. In October 2014, disease recurrence was diagnosed, with bone, lung and lymph node metastasis. Hormone treatment was changed to tamoxifen and trastuzumab was restarted. One year later, due to bone disease progression, she started letrozol and lapatinib. In August 2016, the patient complained about lower limb weakness. MRI of the spine showed a soft tissue mass in L5 with radicular compression. She was evaluated by a neurosurgeon and it was decided there was no surgical indication. Thoracic computed tomography (CT) scan showed lymph node enlargement in right pulmonary hilum and 3 micronodules in both lungs which were stable in relation to previous exams since 2015 (Fig. 1). Echocardiogram was unremarkable, presenting an ejection fraction of 61%. The patient underwent palliative L5-S1 radiotherapy and started on steroids (prednisolone 20mg/day). Seven weeks before admission, she was started on weekly paclitaxel (80 mg/m2) (pretreatment with ranitidine 50 mg endovenous (EV), dexamethasone 8 mg EV and clemastine 2 mg EV) and trastuzumab (600 mg) subcutaneous q21 days.
She reported progressive fatigue and generalized muscular weakness on the fourth week of treatment. Her oncologist increased prednisolone to 30 mg/day. However, she continued getting worse and, in the few days before admission, she was not able to stand or walk without assistance. She had a dry cough, no thoracic pain and had not evaluate fever at home. She had a total of 7 weeks of treatment.
At admission, she was hypotensive (94/56 mmHg), tachycardic (113 bpm), with fever (auricular temperature: 38.1ºC), eupneic, with peripheral saturation in room air of 78%. She was oriented in time and space, had pale skin and mucous membranes and pulmonary auscultation revealed fine crackles in both lung bases. No other relevant alterations were observed.
Arterial blood gas analysis, on a FiO2 21%, showed: pH: 7.48; pCO2: 33.4; pO2: 51.6; SatO2: 87.8%; HCO3: 25.8; Lact: 1.1.
Blood analysis exhibited a normocytic normochromic anemia and reactive C protein elevation (Table 1).
Urianalysis had no signs of infection. Electrocardiogram showed sinus rhythm at 109 bpm. Chest radiography revealed discrete bilateral infiltrates (Fig. 2). An angio-CT scan was executed to rule out pulmonary embolism and showed diffuse bilateral ground-glass opacities, mosaic attenuation pattern and some linear densifications (Fig. 3).
She was admitted in an internal medicine ward. The case was discussed with her oncologist and a pneumonologist. She was treated with empiric antibiotics, 7 days of ceftriaxone and 3 days of azithromycin. Blood cultures were negative. The dose of corticoid treatment was initially increased: methylprednisolone, 62.5 mg/day for three days, then switched to oral prednisolone, 20 mg daily. There was a favorable clinical and analytical response.
Bronchofibroscopy was performed and displayed polypoid lesions in anterior trachea and a lesion in the right superior lobe compatible with breast cancer metastization. Bronchoalveolar lavage (BAL) revealed intense lymphocytosis (50.2%), with minor neutrophilia (7.6%). The CD4/CD8 ratio was slightly diminished (1.18). Cultures for bacterial, mycobacterial and Pneumocystis jiroveci were negative.
She was discharged on the 10th day, asymptomatic and no need of oxygen supplementation. Since there was already a strong suspicion of antineoplastic-induced lung injury she did not resume the same therapeutic scheme. Hormone therapy was restarted, with exemestane 25mg/day, without any HER-2 blockade.
The CT scan was repeated 15 days after the first one and did not show any of the alterations previously reported (Fig. 3).
She was followed in internal medicine consult for 3 months and kept her usual oncology consults, remaining asymptomatic.
Discussion
Interstitial lung disease caused by either paclitaxel (a taxane chemotherapy drug) or trastuzumab (a humanized IgG1 monoclonal antibody) is a rare complication1-2. There are a limited number of reports in the literature of paclitaxel and/or trastuzumab related lung injury4-12. Usually, anti-neoplastic induced respiratory disease presentation includes interstitial pneumonitis, hypersensitivity pneumonitis, interstitial lung fibrosis and organizing pneumonia4-12.
The diagnosis relies on a combination of clinical, radiological and histological findings and is, essentially, an exclusion one3. The perception of a definite temporal association between exposure to the agent and the development of respiratory complaints is key to diagnostic suspicion13.
The clinical manifestations include dry cough, dyspnea, low-grade fever and hypoxemia3. The imaging features of drug-induced injury are nonspecific and include reticular markings, ground glass opacities or consolidations3,14. Drug lymphocyte stimulation test coupled with bronchoalveolar lavage may assist in the diagnosis8,13.
The mechanism of drug-induced interstitial pneumonitis is not well understood, but it may be consequence of a cell-mediated immunologic reaction7,8.
In this case, the patient started treatment with paclitaxel and trastuzumab 4 weeks before the symptoms begin and they were progressively worsening. In the emergency department, a CT scan showed diffuse bilateral ground-glass opacities. Several diagnostic hypotheses were then formulated. The first one was 1) infection, given the fever, cough and elevation of the inflammatory markers, especially, in an immunocompromised patient. She immediately started antibiotics, but BAL showed only a discrete neutrophilia and cultures (blood and BAL) were negative (there is the confounding factor of BAL being done after 5 days of antibiotics). Other differential diagnoses were: 2) pulmonary embolism that was ruled out by the CT scan; 3) cardiogenic edema, but she was not hypervolemic and had a recent normal echocardiography; 4) lung metastasis progression, but the emergency CT did not show an increasing on previously target lesions, any new lesions, nor a pattern compatible with lymphangitic carcinomatosis; 5) radiation-induced lung injury may develop many months or years after RT, but in this case was not likely because, once again, a previous recent CT scan did not show these alterations and the diffuse pattern of the ground glass opacities was not compatible with localized radiation-induced lung injury.
The time association, symptoms and CT scan findings were all compatible with drug-induced interstitial pneumonitis. The lymphocytic predominance of the bronchoalveolar lavage with a low CD4/CD8 ratio also favors the diagnosis3,11,13-15. One of the limitations of this work is that we cannot affirm which of the drugs was responsible for this reaction. The drug lymphocyte stimulation test is not available in the center and is not fundamental to reach a diagnosis, but could have helped in this case to identify the culprit agent.
There are reports of delay interstitial pneumonitis caused by each drug. We could even question if the synergistic efficacy they present against the tumor could be reproduced in terms of side effects4 and this was caused by both drugs. Other thing we can’t fully explain is the fact that she had already been treated, years before, with a taxane (docetaxel) and trastuzumab, simultaneously, and no adverse reaction was reported. We know that a rechallange with a suspected drug for interstitial pneumonitis is possible and, in most of the cases, safe.
Having a high diagnostic suspicion, the antineoplastics were suspended, corticoid dose was increased and the patient improved. Furthermore, a CT scan performed 15 days after discharge showed complete resolution of the ground glass opacities, reiterating the diagnosis.
The cornerstone treatment of drug induced lung disease is the discontinuation of the drug. In this case, as we did not know which drug was responsible, both treatments were interrupted.
The evidence to support benefit of glucocorticoids in the setting of drug-induced lung toxicity is largely observational3. Thus, for patients with stable or improving disease, glucocorticoids are generally withheld and an expectant attitude is assumed. On the other hand, empiric glucocorticoid therapy is usually initiated in patients with severe disease (grade 3 or 4 according to the National Cancer Institute common toxicity criteria)3, as was in this case of grade 3 pneumonitis.
Conclusion
Antineoplastic-induced interstitial lung disease is a rare complication, but it adds significant morbidity and mortality risk to cancer patients. Early diagnosis may lower that risk by prompt discontinuation of the antineoplastic drug. The diagnosis is, mainly, based on a time association between the oncological therapy and symptoms. It is not a frequent cause of respiratory insufficiency in internal medicine wards. Such report calls attention to this adverse effect, hopefully, leading to an increase the diagnostic suspicion and better outcomes.
Quadro I
Blood results at admission
| Parameter | Result | Normal value |
| Hemoglobin | 11.2 g/dL | 12.0 – 16.0 g/dL |
| Leucocytes | 4.65 x 103/uL | 3.6 – 11.0 x 103/uL |
| Neutrophils | 3.68 x 103/uL | 1.3 – 8.8 x 103/uL |
| Lymphocytes | 0.61 x 103/uL | 1.0 – 4.8 x 103/uL |
| Platelets | 262 x 103/uL | 150 – 440 x 103/uL |
| Urea | 36 mg/dL | 17 – 50 mg/dL |
| Creatinine | 0.67 mg/dL | 0.51 – 0.95 mg/dL |
| Reactive C protein | 13.72 mg/dL | 0 – 0.5 mg/dL |
Table 1: Blood analysis results at the admission to the emergency department.
Figura I
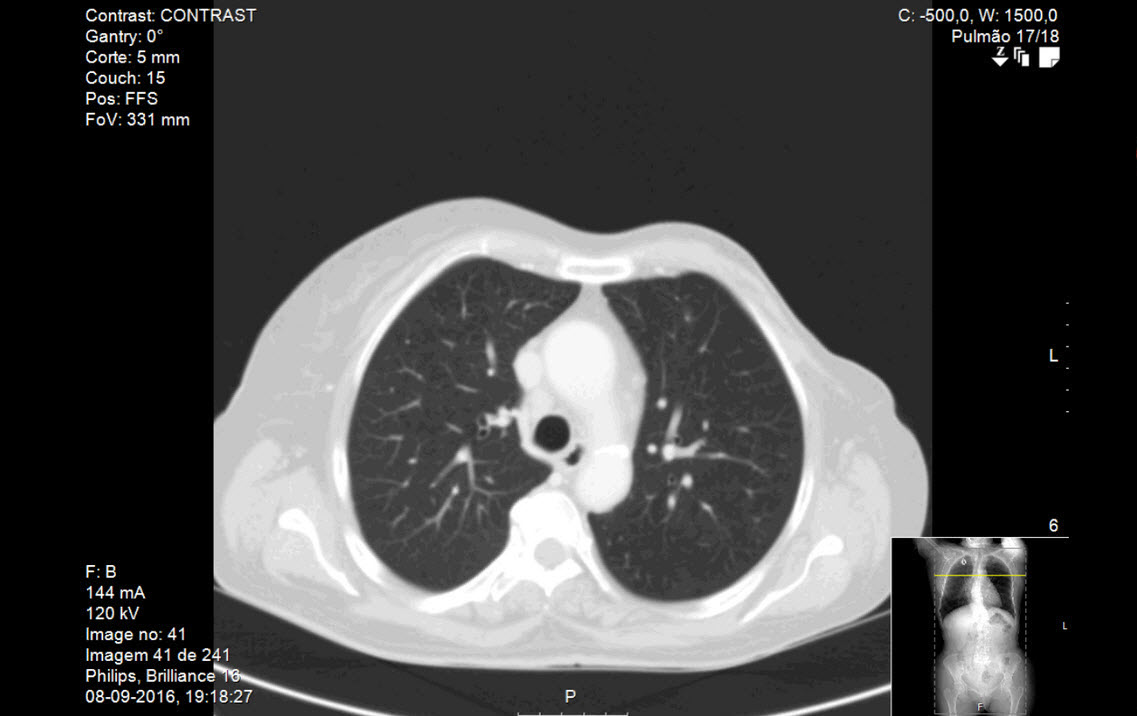
Figure 1: Thoracic computed tomography (CT) executed before beginning of treatment with paclitaxel and trastuzumab. There is no evidence of interstitial lung disease. The complete CT (not exhibited) showed lymph node enlargement in right pulmonary hilum; three micronodules: one with 6 mm in the anterior segment of the right superior lobe, other with 10 mm in the superior segment of the left inferior lobe and the third with 3 mm in the left apical posterior segment, all stable in relation with previous exams (not exhibited). No other relevant alterations were observed.
Figura II
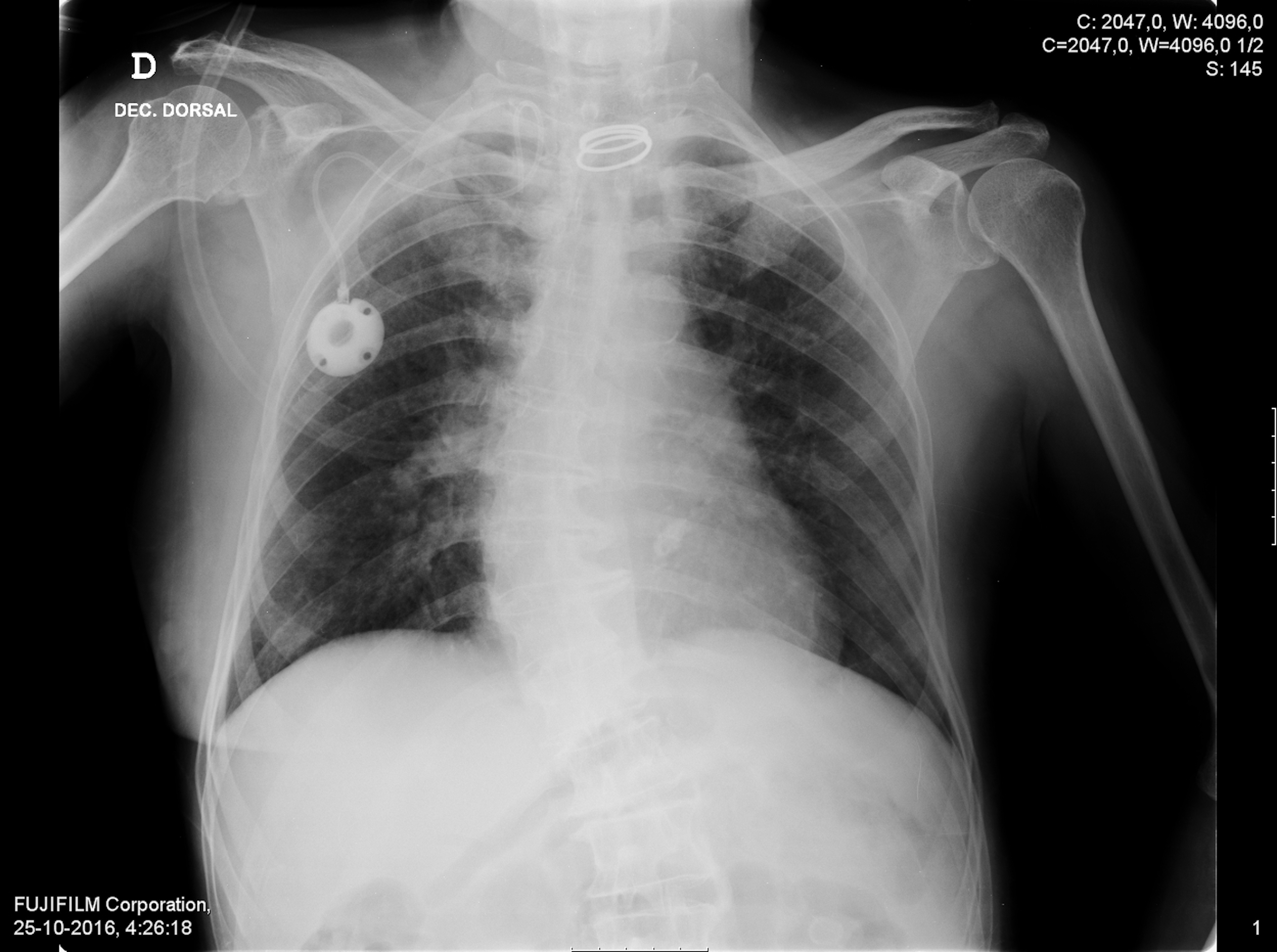
Figure 2: Chest radiography at the emergency department revealed discrete bilateral infiltrates.
Figura III
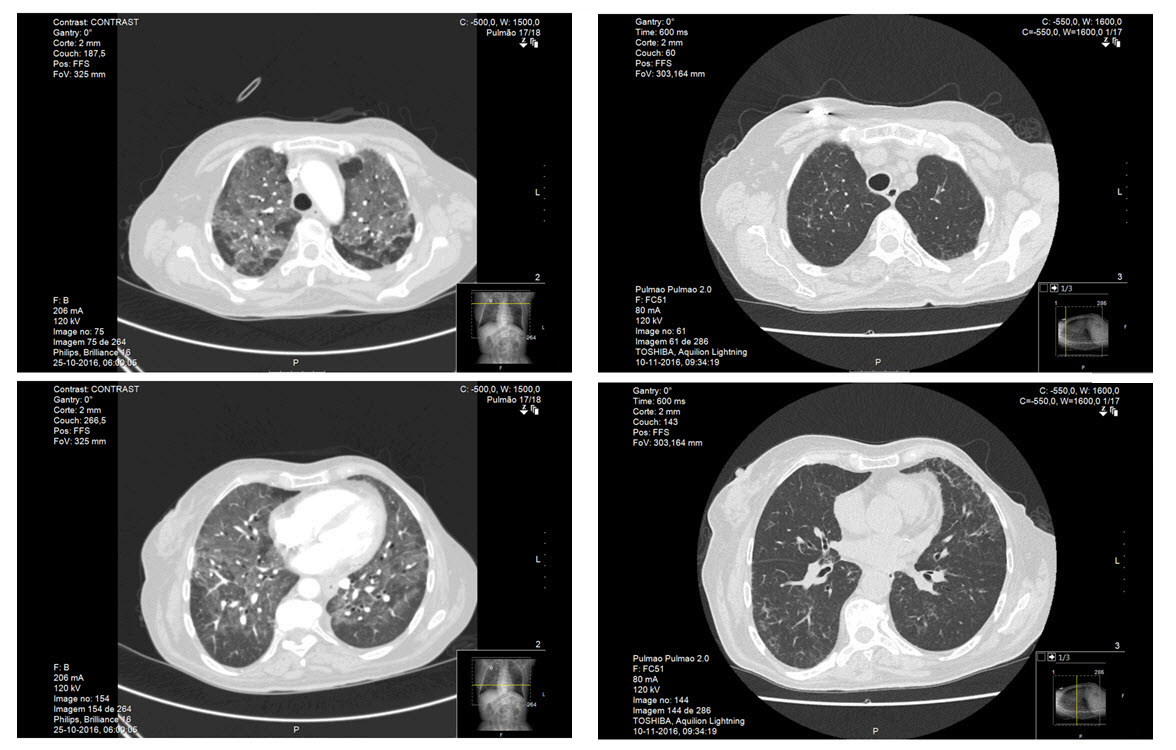
Figure 3: Left: Thoracic angio - computed tomography (CT) at the emergency department showing extensive diffuse bilateral ground-glass opacities, mosaic attenuation pattern and some linear densifications. Right: Thoracic CT executed 15 days after hospital discharge and suspension of anti-neoplastic drug showed complete resolution of the ground glass opacities. The complete exam (not exhibited) maintains the 3 micronodules previous reported with the same size and characteristics.
BIBLIOGRAFIA
1. Paclitaxel product monography [accessed 2017 January 2]. Available in: https://www.uptodate.com/contents/paclitaxel-conventional-drug-information?source=search_result&search=paclitaxel&selectedTitle=1~150.
2. Rituximab product monography [accessed 2017 January 2]. Available in: https://www.uptodate.com/contents/trastuzumab-drug-information?source=search_result&search=trastuzumab&selectedTitle=1~98.
3. Maldonado F, Limpe AH, Jett JR. Pulmonary toxicity associated with systemic antineoplastic therapy: Clinical presentation, diagnosis, and treatment. In UpToDate 2016 [accessed 2017 January 2]. Available in: https://www.uptodate.com/contents/pulmonary-toxicity-associated-with-systemic-antineoplastic-therapy-clinical-presentation-diagnosis-and-treatment?source=search_result&search=pulmonary%20toxicity%20associated%20with%20systemic%20antineoplasic%20therapy&selectedTitle=2~150
4. Abulkhair O, Melouk WE. Delayed Paclitaxel- Trastuzumab-Induced Interstitial Pneumonitis in Breast Cancer. Case Rep Oncol 2011;4:186–191
5. Kuip E, Muller E. Fatal pneumonitis after treatment with docetaxel and trastuzumab. Neth J Med.2009;67(6):237-9.
6. Kim S, Tannok I, Sridhar S, Seki J, Bordeleau L. Chemotherapy-induced infiltrative pneumonitis cases in breast cancer patients. J Oncol Pharm Pract.2012;18(2):311-5.
7. Nagata S, Ueda N, Yoshida Y, Matsuda H, Maehara Y. Severe interstitial pneumonitis associated with the administration of taxanes. J Infect Chemother.2010;16(5):340-4.
8. Ostoros G, Pretz A, Fillinger J, Soltesz I, Dome B. Fatal pulmonary fibrosis induced by paclitaxel: a case report and review of the literature. Int J Gynecol Cancer.2006;16 Suppl 1:391-3.
9. Pepels MJ, Boomars KA, van Kimmenade R, Hupperets PS. Life-threatening interstitial lung disease associated with trastuzumab: case report. Breast Cancer Res Treat.2009;113(3):609-12.
10. Radzikowska E, Szczepulska E, Chabowski M, Bestry I. Organizing pneumonia caused by trastuzumab (Herceptin) therapy for breast cancer. Eur Respir J.2003;21(3):552-5.
11. Taus-García A, Sánchez-Font A, Servitja-Tormo S, Pijuan L, Maiques-Llácer JM, Curull V. Arch Bronconeumol. 2010;46(8):442-44.
12. Bettini AC, Tondini C, Poletti P, Caremoli ER, Guerra U, Labianca R. A case of interstitial pneumonitis associated with Guillian-Barré syndrome during administration of adjuvant trastuzumab. Tumori.2008;94(5):737-41.
13. Camus P, Annlyse F, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration.2004;71(4):301-26.
14. King, Jr TE. Role of bronchoalveolar lavage in diagnosis of interstitial lung disease. In UpToDate 2016 [accessed 2017 January 2]. Available in: https://www.uptodate.com/contents/role-of-bronchoalveolar-lavage-in-diagnosis-of-interstitial-lung-disease.
15. Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM. An Official American Thoracic Society Clinical Practice Guideline: The Clinical Utility of Bronchoalveolar Lavage Cellular Analysis in Interstitial Lung Disease. Am J Respir Crit Care Med 2012; 185(9):1004–14.

