Introduction
Renovascular hypertension is the most frequent, potentially correctable, type of secondary hypertension, with a prevalence of up to 5% of all hypertensive adult patients1–3. The hyponatremic hypertensive syndrome (HHS) was first reported in 1952 (case report)1,3,4and subsequently characterized (1965 by Brown et al.) as a combination of hyponatremia (serum sodium <136 mmol/L), hypertension (systolic blood pressure >165 mmHg and diastolic >95 mmHg, with or without antihypertensive medication) and evidence of renal ischemia1,5,6.
It is found in medical literature mostly as anecdotal isolated cases, with 3 small case series described (with 4, 6 and 32 patients)4,6,7. Despite the rarity of clinical reports, most authors believe that the syndrome is underdiagnosed, with no incidence value readily apparent in the literature4–8. From the small series of cases, it is depicted as been more frequent in asthenic elderly women, usually with a heavy smoking history3,4,6,8.
The pathophysiology of the syndrome is complex and it is not yet fully understood. Most likely it results from a combination of factors, based on the unilateral kidney ischemia (usually attributed to unilateral renal artery stenosis). Ischemia promotes elevation of renin levels, (pathognomonic finding in HHS) and stimulates the renin-angiotensin system (RAS) with high concentrations of angiotensin II, leading to hypertension and water/electrolyte loss by pressure natriuresis, despite elevation of the antidiuretic hormone (ADH), responsible for the polydipsia2,4–9.
Most patients present with central nervous system symptoms, such as confusion or headache, and also polydipsia, polyuria, weight loss and postural dizziness. Other symptoms related to severe hypertension and/or hyponatremia/hypokalemia can also be present4,7. Identification of the syndrome is crucial for correct treatment of the underlying cause, oftentimes curable. When left untreated, HHS has a poor prognosis with mortality rates as high as 25%4,6.
Case Report
A 74-year-old man, previously independent, presented to the emergency department with nausea, vomits, diarrhea, prostration and episodic confusion. At admission, the patient was disoriented and unable to provide clinical information. A friend revealed that the gastrointestinal symptoms had started that day but the disorientation episodes had begun a few days earlier. There was no reported fever.
The patient had a past medical history of hypertension (usual blood pressure (BP) level of 220/120 mmHg), with poor therapeutic adherence as he did not take any medications despite of medical prescriptions of lisinopril and amlodipine. He was a former smoker and had a history of alcohol abuse, Madelung disease, bilateral Dupuytren contracture and a prior 15-year-old subdural hematoma after a car accident.
Examination showed an emaciated man with disperse large lipomas on the anterior and posterior cervical regions, anterior pectoral girdle and left shoulder. The liver was soft and smooth, palpable 2 cm below the costal margin. Collateral liver circulation was notorious at the abdominal examination, as well as bilateral Dupuytren contracture. There was no abdominal bruit or peripheral edema. No other important sign was present. During physical examination, the patient presented a tonic-clonic seizure which lasted 30 second and BP dropped to 82/50 mmHg.
Laboratory results showed severe hyponatremia of 116 mmol/L (reference range (RR) 136–146 mmol/L), hypokalemia of 2,8 mmol/L (RR 3,5-5,1 mmol/L), creatinine 1,10 mg/dL (RR 0,72-1,18 mg/dL), estimated glomerular filtration rate (Cockroft-Gault equation) of 58,33 mL/min/1,73m2, bicarbonate 27,8 mmol/L (RR 21-29 mmol/L), pH 7,54 (RR 7,35-7,45), serum osmolality 235 mosm/kg (RR 260–302 mosm/kg), blood alcohol concentration of 0,00 g/L, c-reactive protein 1,53 (RR <0,5 mg/dL) and leukocytes 16,0 G/L. Head computed tomography (CT) scan only revealed lesions compatible with prior trauma and subdural hematoma drainage.
The patient was admitted with the diagnosis of severe symptomatic hyponatremia and hypertension, starting electrolyte correction immediately. As blood electrolyte correction proceeded, the patient became more oriented, confirming the history of hypertension and referring polyuria as well. No more seizures occurred during the hospital stay and BP climbed to the usual levels so nifedipine 30 mg was started, and secondary hypertension study was performed.
The patient´s renal ultrasound revealed a right kidney length of 6,2 cm and a normal left kidney length, with normal corticomedullary differentiation. The right renal artery was not possible to assess using color Doppler, and it suggested an increased resistance in the left renal artery. Angio-CT scan revealed an atrophic right kidney with stenosis of the entire length of the right renal artery and no abnormalities on the left artery (Fig. 1 and 2).
Accordingly, an extremely increased renin plasma level of 655 uU/mL (RR 7-76) was detected, with no relevant changes in the levels of aldosterone, catecholamine secretion and thyroid hormones. No urine osmolality or electrolyte assessment in a 24-hour urine collection (before electrolyte correction) were obtained, because the patient presented with seizures and treatment with hypertonic fluid was started immediately.
Renal scintigraphy showed a right kidney function of only 2% (Fig. 3). Arteriography was performed, but angioplasty was not possible due to the small right vessel caliber (Fig. 4).
A right laparoscopic total nephrectomy was scheduled, as the hyponatremia and hypokalemia were impossible to be fully corrected without continuous intravenous fluid therapy. After surgery, blood electrolytes and BP normalized, with no need for electrolyte supplements or BP medication.
Discussion
This patient was geriatric and had a prior smoking history, factors known to be associated with the development of atherosclerotic renovascular disease4,6. Since the pathophysiology of the HHS is poorly understood, it seems most likely that the development of a vicious cycle (hypertension - pressure natriuresis – increase activation of the RAS) is based on a “critical” arterial renal obstruction, with relatively recent onset, alongside the other kidney with normal function3,4,6. This critical arterial obstruction tends to be of atherosclerotic origin in adults and caused by fibromuscular dysplasia in the rare case reports of young children with HHS6.
The initial treatment depends on the clinical presentation6. As our patient presented with severe symptomatic hyponatremia, the initial focus of the therapy was to obtain neurological stability. As he became asymptomatic, antihypertensive treatment was started. Regardless of the antihypertensive treatment being the cornerstone for the HHS treatment6, the truth is that response to surgery, angioplasty or drug therapy cannot be ascertained from case series, case reposts or literature at the present time, as the information is still scarce3. Considering this limitation, some authors still propose that treatment for HHS should mandatorily involve revascularization of the kidney in cases of bilateral renal artery stenosis (presumed to be critical in one kidney4), stenosis in solitary functioning kidneys and patients than do not respond to medical management3.
Medical management includes smoking cessation, controlling BP and cholesterol levels, aspirin and folate administration, exercise and balanced diet3. Considering the BP treatment, drugs that are less likely to stimulate the renin-angiotensin system are preferable, since angiotensin-converting-enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are associated to an increased risk of acute renal failure. Therapeutic options relay on calcium channel inhibitors (with a minimal effect in RAS) and beta-adrenergic blockers (that may suppress renin release)6.
Percutaneous transluminal angioplasty (PTA) and surgical revascularization may allow to preserve renal function and eliminate or reduce a lifelong need for BP treatment. Nephrectomy can be considered as an alternative when the kidney presents less than 10% of the total renal function6. The right kidney of our patient was small, atrophic and only contributed with approximately 2% of the renal function. As it was impossible to fully compensate the electrolyte balance of the patient without continuous intravenous fluids, a nephrectomy was performed. At discharge the patient presented normal electrolyte balance and controlled BP, with no need of electrolyte supplementation or antihypertensive medication.
HHS was cured in our patient but lacking the correct identification of the syndrome is a cause of its high mortality (up to 25% in adults)6. Increasing awareness of HHS is imperative to correctly identify and effectively treat patients. Increasing awareness will also enable further investigation on this syndrome and clarification of its pathophysiological mechanisms.
Acknowledgements
We gratefully acknowledge and thank Dr. João Casalta Lopes for his expertise and skillful technical assistance.
Figura I
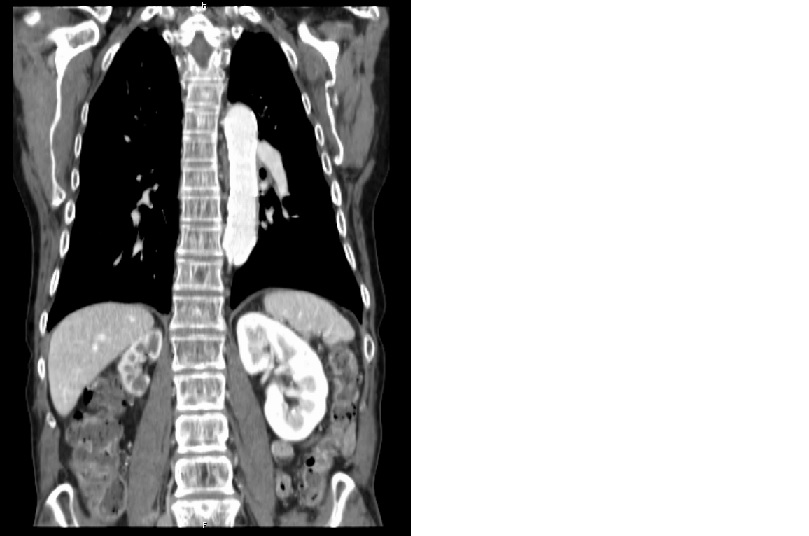
Figure 1 - Angio-CT scan revealed an atrophic right kidney with stenosis of the entire length of the right renal artery and no abnormalities on the left artery.
Figura II
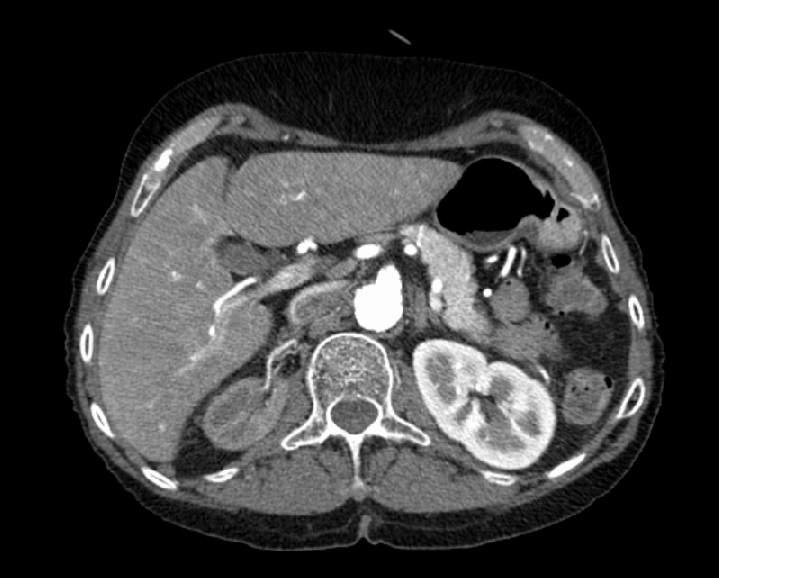
Figure 2 - Angio-CT scan revealed an atrophic right kidney with stenosis of the entire length of the right renal artery and no abnormalities on the left artery.
Figura III
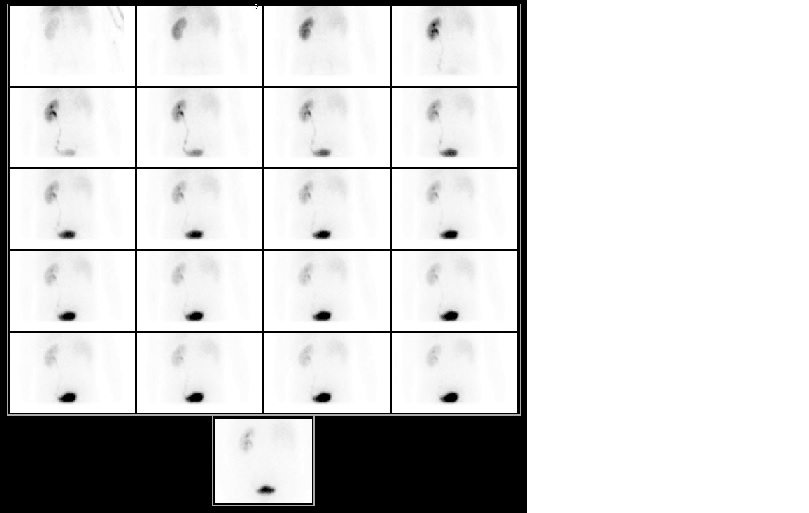
Figure 3 - Renal scintigraphy showed a right kidney function of only 2%.
Figura IV
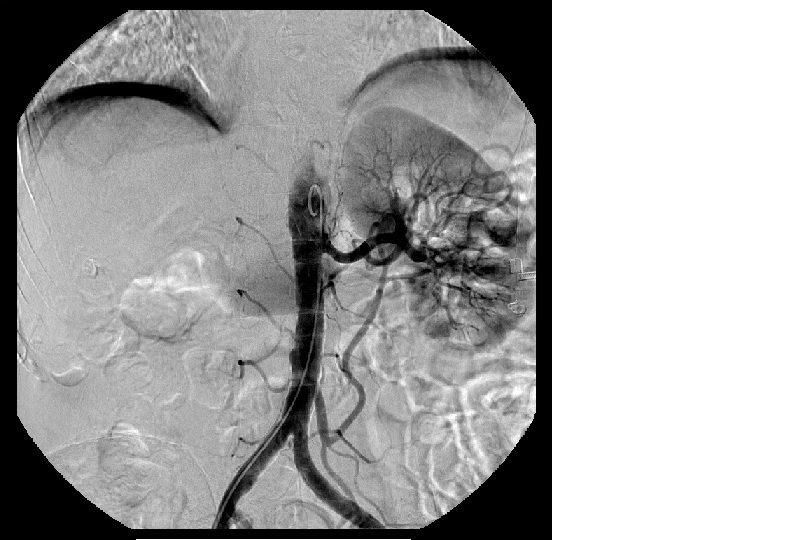
Figure 4 - Arteriography was performed, but angioplasty was not possible due to the small right vessel caliber.
BIBLIOGRAFIA
References
1. Report AC. Hyponatremic – Hypertensive Syndrome Associated With Renovascular Hypertension. 2002;66(March):297–301.
2. Lee SY, Lau H. Effectiveness of unilateral nephrectomy for renal hypertension in adults. Asian J Surg. Asian Surgical Association; 2008;31(4):185–90.
3. Saeed K.; Hyponatremic hypertensive syndrome in an obese man with renal ischemia. Saudi J Kidney Dis Transpl. 2006;17(3):390–4.
4. Agarwal M, Lynn KL, Richards AM, Nicholls MG. Hyponatremic-hypertensive syndrome with renal ischemia: an underrecognized disorder. Hypertension. 1999;33(4):1020–4.
5. Pillai U, Mandakapala C, Mehta K. Resistant hyponatremia and hypokalemia treated successfully with nephrectomy. Clin Kidney J. 2012;5(1):68–9.
6. A. P-A. Pediatric hyponatremic hypertensive syndrome. Therapy. 2005;2(2):301–9.
7. Browne WL, Nair B. The hyponatremic hypertensive syndrome in renal artery stenosis: an infrequent cause of hyponatremia. J Postgrad Med. 2007;53(1):41–3.
8. Dixit MP, Hughes JD, Theodorou A, Dixit NM. Hyponatremic hypertensive syndrome (HHS) in an 18-month old-child presenting as malignant hypertension: a case report. BMC Nephrol. 2004;5:5.
9. Machado V, Pontes T, Mota T, Afonso AC. Síndrome hipertensiva hiponatrémica em criança de quatro anos. Acta Pediátrica Portuguesa. 2007;82–4.





