CASO CLÍNICO
A 58-year-old man presented to the emergency department with a 10 days history of jaundice, itching, dark urine and light stools. He denies having fever or flu-like symptoms, abdominal pain, vomiting or weight loss. He was diagnosed with a right colon adenocarcinoma in 2012. A hemicolectomy was performed followed by chemotherapy regimen with oxaliplatin, leucovorin, and 5 FU 19 months before. The follow-up CT-scan, one month before emergency admission, revealed an increased portal vein diameter (15 mm), tortuous splenic veins and a slight splenomegaly, with no residual neoplastic disease evidence. Thirteen days earlier the beginning of symptomatology, he was medicated with a 7-days course of amoxicillin-clavulanate for an abdominal wall hematoma. He denies using other than his usual anti-hypertensive drugs, over-the-counter medication, as well as illicit intravenous-drugs or alcohol abuse. There was no sexual risk behavior or recent travels. His vaccination plan was updated. He had 2 daughters with type 1 glycogenosis, one submitted to liver transplant. On examination, the patient was icteric. His heart rate was 65 ppm, temperature 36.6oC and blood pressure 141/61mmHg. Cardiopulmonary examination was unremarkable. There was no abdominal tenderness or signs of chronic liver disease.
Baseline full blood count revealed haemoglobin 12.5g/dL, with normal leucocytes and platelets. His liver test results were total bilirubin 13.96mg/dL (direct fraction 11.82mg/dL), aspartate aminotransferase 4xULN, alanine aminotransferase 6xULN, alkaline phosphatase 4xULN, γ-glutamyltransferase 7xULN. Albumin and coagulation tests were normal. Tests for hepatitis viruses (HBV, HCV, HIV, EBV, CMV), auto-antibodies (ANA, AMA, SMA, LKM, LC1, SLA/LP) and metabolic diseases such as hereditary hemochromatosis and Wilson’s disease, were negative. Carcinoembryonic antigen was normal. Abdominal ultrasound and colangio-MNR show normal intra and extra-hepatic biliary tract and confirmed portal vein ectasia (15mm) and moderate splenomegaly (140mm). Upper GI tract endoscopy was unremarkable for signs of portal hypertension. Liver biopsy showed centrilobular cholestasis and portal inflammation; and areas of intra-sinusoidal collagen deposition without sinusoidal rupture or fibrous obliteration of the central vein lumen (Fig.1).
As Good Medical Practices advocate, before thinking about DILI, the commonest causes of intra-hepatic cholestasis must be ruled out. Infectious, metabolic, alcoholic and non-alcoholic, and autoimmune hepatitis were excluded. Type 1 glycogenosis is an autosomal recessive disease that usually manifests in the neonatal period or childhood and heterozygote carriers are assymptomatic.
The authors could understand this case in two different ways. On one hand, the evolution of disease could be initially analyzed as a continuum manifestation of a hepatotoxicity that eventually the patient acquired after chemotherapy (Fig.2A). In our patient, the criteria of portal hypertension with splenomegaly de novo suggested hepatotoxicity by oxaliplatin. On the other hand, time had demonstrated that a clinical course with a climax, supported by an additional noxious factor, could be a more reasonable theory. Amoxicillin-clavulanate (represented by a dotted line in Fig.2B), a well-known hepatotoxic drug could also represent a potential trigger to the exacerbation of the hidden underlying effect that was inducing by oxaliplatin from the beginning.
No specific therapy was instituted besides suspension of amoxicilin-clavulanate and introduction of ursodeoxycholic acid (UCDA). Hydroxyzine was used for symptomatic relief of pruritus. During the admission time patient show no signs of encephalopathy, total bilirubin was progressively increasing, without evidence of coagulopathy. He was discharged 22 days later with total bilirubin 25.03mg/dL with a 3-times week blood test and medical visiting. Ten weeks after supportive treatment and UDCA, hyperbilirubinemia decreased to 2.49 mg/dL. Fourteen months after, our patient did not present splenomegaly or other signs of portal hypertension in abdominal doppler and CT-scan.
DISCUSSÃO
A CASE OF DOUBLE DILI
DILI is applied to any toxicity to the liver by a drug, herbal or dietary supplement. It could mimic any form of liver disease which may be predictable and dose-dependent only in few cases. In fact, the majority is based on idiosyncratic or hypersensitive reactions.1 Various factors such as age, gender, dose or co-administered drugs may increase the risk to develop DILI.1 Because there are no specific findings or laboratory tests that definitely prove the suspicion, even liver biopsy, DILI is a diagnosis of exclusion and relies upon clinical judgment, knowledge about the potential of the harmful factor to cause liver injury and a careful drug history.2 Acute drug-induced cholestatic injury represents one of the three major forms of DILI and has a better prognosis than hepatocellular injury. In most cases, the natural course is followed by a normalization of serum liver tests within 3 months after stopping the drug and the outcome is, generally, good.1 This case is an interesting example of two different forms of DILI by two different drugs, in different times, in the same patient. Our case report a 58-year-old man without significant epidemiological risk, clinical stigmas or laboratory alterations for previous acute or chronic hepatic disease. After chemotherapy with CAPEOX and FOLFOX 6, he began to show persistent asymptomatic changes liver test supporting a cholestatic pattern and moderate thrombocytopenia. Seven months after the last chemotherapy session, it is recognized de novo imaging alterations of portal hypertension. Then, 7 months after chemotherapy, maintaining those slight laboratory changes, he was exposed to amoxicilin-clavulanate and develop an icteric cholestasis with severe hyperbilirubinemia.
DILI BY AMOXICILIN/CLAVULANATE
Amoxicillin-clavulanate is a widely prescribed.3,4 Liver damage by amoxicillin-clavulanate embrace 1.7/100000 prescriptions and the most prevalent lesions are cholestasis pattern. Symptoms could begin in any period after the end of the treatment, but typically after 4-10 weeks. There are some criteria of poor prognosis, as severe hyperbilirubinemia, liver function compromised and encephalopathy.4 Histopathological examination could demonstrate centrilobular or panlobular cholestasis, lymphocytic or necrosis of ductal epithelial cells or even ductopenia.4 Actually, in judging the likelihood of acute DILI by amoxicillin-clavulanate some features were indispensable to hold on this hypothesis: time to onset, time to recovery, the clinical pattern, the exclusion of other causes and the known potential hepatotoxicity of the drug. Because of the unpredictable course of this case, it was important to manage it with liver transplantation unity. The maximum value for hyperbilirubinemia was 26.70 mg/dL; the higher hyperbilirubinemia, the most probably to develop hepatic dysfunction, but in any occasion it was evident in our patient. Despite hyperbilirubinemia, our patient not meet other criteria of poor prognosis. Liver biopsy is usually not required and may demonstrated portal inflammation with bile-duct injury, vacuolar degeneration and necrosis. The clinical and medication history, the suggestive biopsy findings and causality scales give a high probability of drug hepatotoxicity (Fig.3). As mentioned in literature, there is no effective treatment for DILI cholestasis, except discontinuation of the offending agent and supportive care. UDCA or corticosteroids are regarded as experimental due to lack of adequate controlled trials.1 It also requires longitudinal monitoring of patient and laboratory values during a period of time to observe the evolution. Before the 3 months cut off referred by Literature, bilirubinaemia decreased ten times in our patient and the remaining liver test progressively become normal (Fig.4).
DILI BY OXALIPLATIN
Oxaliplatin-based chemotherapy regimens are presently standard of care for the treatment of colorectal cancer in both the adjuvant treatment and metastatic disease setting, leading to significant improvement in response rate, disease-free and overall survival. DILI oxaliplatin-induced is rarely fatal and causes morphological lesions involving hepatic microvasculature which can lead to a widely spectrum liver changes, such as sinusoidal obstruction syndrome, nodular regenerative hyperplasia, non-cirrhotic portal hypertension.4,5 It was firstly reported by Rubbia-Brandt et al in 2004. Many studies have confirmed the association of oxaliplatin with sinusoidal injury, reporting rates of injury from 19% to 78%.4,6 Pathophysiology is still not completely understood, but histopathologic changes by DILI oxaliplatin-induced share similarities to the changes seen in the sinusoidal obstruction syndrome.6 Sinusoidal dilatation, congestion and centrilobular necrosis, which could be not clinically apparent in acute phase, are some of the main changes documented.2 Non-cirrhotic portal hypertension caused by oxaliplatin is frequently reported. Eighty six percent have an increase in spleen size and 24% develop splenomegaly. Many authors suggest that the increases in spleen size may serve as a potential biomarker for predicting hepatic sinusoidal injury.6 Nalbantoglu et al reviewed that most patients has recovered without complications during 93 months follow-up, indicating that these lesions are reversible.
The real understanding of this case have become clarify over time. The elegance of this report relies on the details previous to the ingestion of amoxicillin-clavulanate which allowed the diagnosis of a double-DILI. In our patient, after other important concurrent causes were excluded, the criteria of non-cirrhotic portal hypertension with splenomegaly de novo suggested hepatotoxicity by oxaliplatin. During follow up patient has never develop any signs of clinical disease. The timing of portal hypertension resolution was consistent with literature.
To our knowledge this is the first reported case of double DILI by amoxicillin-clavulanate and oxaliplatin. It is paradigmatic of how uncertain is the cross sensitivity to hepatic injury between drugs. Current knowledge about DILI risk factors is not consensual. However, some studies point out age above 55 years old, male gender, long-term treatment and dosis of drug used as possible red flags. But, undoubtedly only clarity of clinical judgment is able to suspect from idiosyncrasy.
Figura I
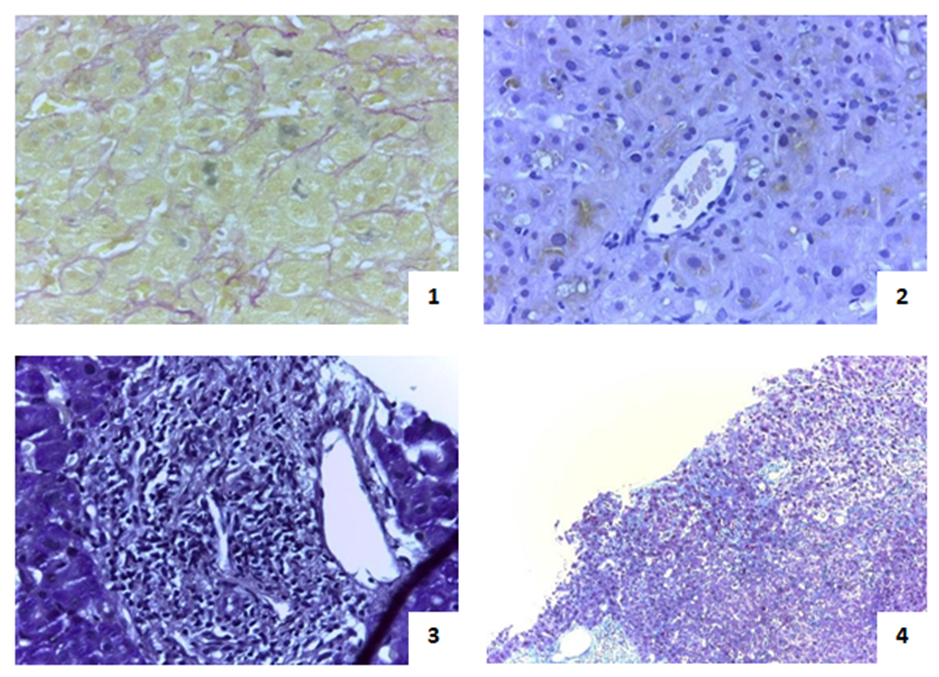
1. Perivenular cholestasis (Hematoxylin-eosin stain, 400x); 2. Intracanicular pigment (Fusher strain, 400x); 3. Portal space with inflammatory infiltrate and hepatitis lesions - details: vein, artery and biliar canaliculus (PAS strain, 400x); 4. Collagen deposition in sinusoids - sinusoidal fibrosis (Masson´s trichrome strain, 100x).
Figura II
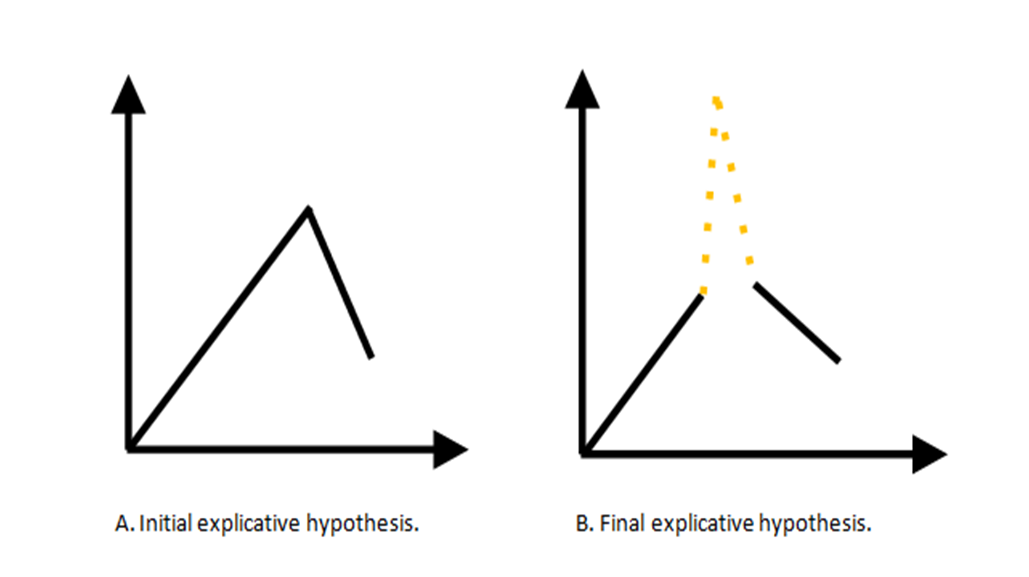
Models of understanding liver changes
Figura III
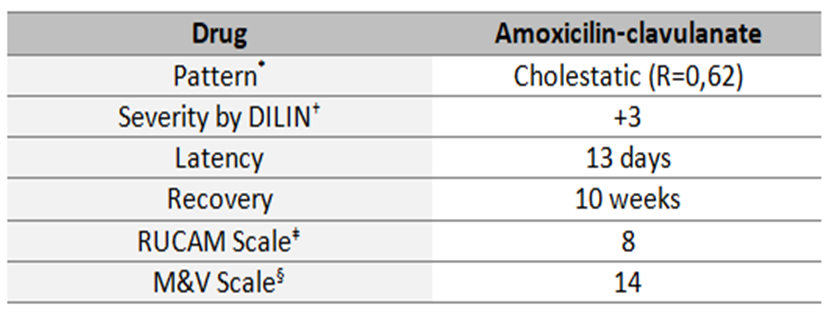
Features of likelihood hepatotoxicity by amoxicillin-clavulanate in this case. *Cholestatic pattern is defined by a ratio (R) between serum enzyme elevations (ALT) and alkaline phosphatase which should be <2. †DILIN, Drug- Induced Liver Injury Network developed a five point scale for grading the severity of DILI. ‡RUCAM, Roussel Uclaf Causality Assessment Method. §M&V Scale, Maria & Victorino (M & V) Scale.
Figura IV
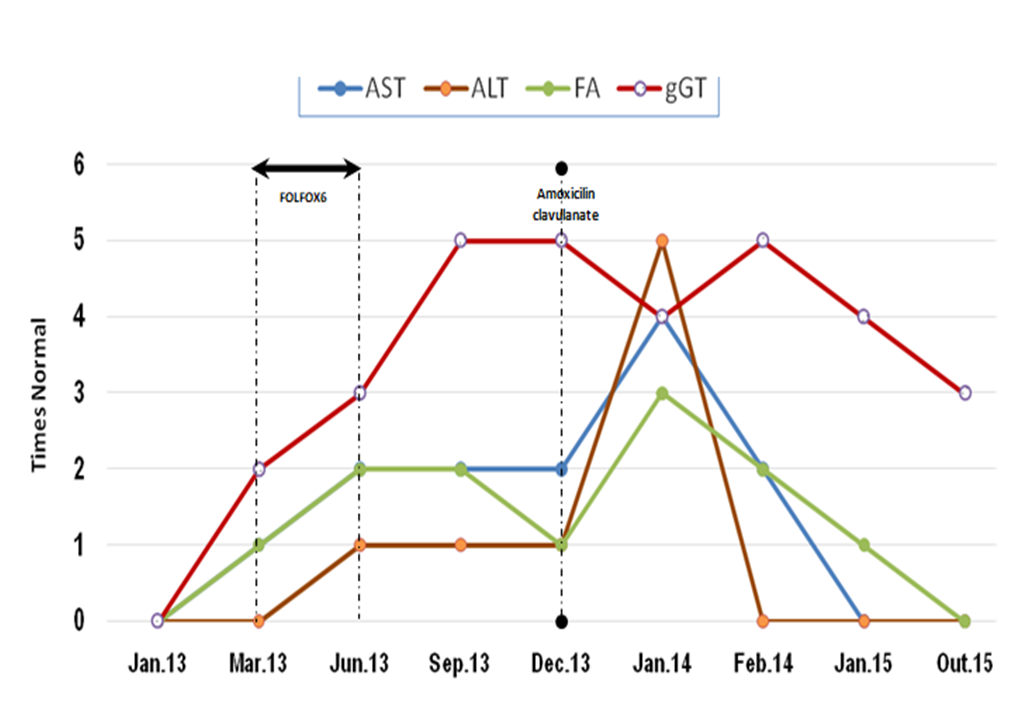
Evolution of laboratory liver test during DILI reactions.
BIBLIOGRAFIA
1European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. Journal of Hepatology. 2009. 51: 237–267.
2Livertox: Clinical and Research Information on Drug-Induced Liver Injury [Internet], National Library of Medicine and National Institute of Diabetes and Digestive and Kidney Diseases. 2015 [cited 2015 Oct 30]. Available from: http://livertox.nih.gov/.
3Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clinic Proc. 2014. Jan;89 (1): 95-106.
4 L. Rubbia-Brandt, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004. Mar;15 (3): 460–6.
5Nalbantoglu IL, Tan BR Jr, Linehan DC, Gao F, Brunt EM. Histological features and severity of oxaliplatin-induced liver injury and clinical associations. J Dig Dis. 2014. Oct; 15 (10): 553-60.
6Overman MJ, Maru DM, Charnsangavej C, Loyer EM, Wang H, Pathak P et al. Oxaliplatin-Mediated Increase in Spleen Size As a Biomarker for the Development of Hepatic Sinusoidal Injury. J Clin Oncol. 2010. May; 28 (15):2549-55.





