Introduction
Pasteurella is a Gram-negative bacteria with a worldwide distribution being primarily commensals of animals.1
In humans, they are known to cause zoonotic infections which have been described from Pasteurella multocida (the most commonly isolated), Pasteurella canis, Pasteurella dagmatis and Pasteurella stomatis.2Human pasteurellosis results from dog or cat bites although licks from these animals have also been associated with infection.2Pasteurella species are a rare cause of infective endocarditis.1 After extensive bibliographic research, we have not found any previous case report of Pasteurella canis bacteraemia with native valve endocarditis.
We present a case of a 61-year-old man, electrician, with previous history of moderate aortic insufficiency due to discrete dilation of the aortic root and ascending aorta, asymptomatic, followed regularly by cardiologist.He presented in the emergency room with a history of shivering for three days. He also referred fatigue, night sweats, anorexia and loss of 12% of his usual body weight over the last two months.Two weeks before he presented purpuric lesions in both lower limbs which disappeared after two days. There were no other symptoms referable to other systems.He performed dental treatments four months before without antibiotic prophylaxis. Regarding the epidemiological context, we emphasize a rural environment with close contact with a dog and a cat, with no history of bites.
On examination, the patient was conscious, pale, with the following vital signs: auricular temperature of 39,8ºC, respiratory rate of 24 breaths/min, peripheral oxygen saturation 98%, blood pressure 121/64 mmHg and heart rate of 96 beats/min. The lung auscultation was normal and cardiac auscultation revealed an aortic diastolic murmur. Electrocardiogram revealed a sinus rhythm. There were no obvious skin lesions.
Workup revealed leucocytes of 9.4 × 109/L (neutrophilia of 86,8%), hemoglobin of 11,0 g/dL (normocytic and normochromic anemia), urea 59 mg/dL and creatinine of 1,3 mg/dL, C-reactive protein of 9,82 mg/dL, aspartate transaminase 75 UI/L, alanine transaminase 100 UI/L, lactic desidrogenase 399 UI/L. Chest radiography and abdominal echography were normal.Two blood cultures were collected and empirical antibiotic therapy with ceftriaxone (2 g/day, intravenously) was established. Two more blood cultures were collected 12h after the first ones.
The patient was admitted to the Internal Medicine ward by fever and constitutional symptoms with two months of evolution.
The transthoracic echocardiography showed a fibrosed tricuspid aortic valve with severe regurgitation and fibrosed mitral valve with slight degree regurgitation, with no signs of endocarditis. On the eighth day of hospitalization, he performed transesophageal echocardiography that showed slight aortic valve ring dilatation (25 mm) with prolapse of part of the right coronary cusp with moderate to severe regurgitation (Figure 1). In the left ventricular outflow tract (in the region of mitral-aortic intervalvular fibrosis) two freely moving vegetations were found (4 and 3 mm), that were not visible, one year earlier, in a transesophageal echocardiogram.Remaining workup was negative including screening for the human immunodeficiency virus. Fever resolved after four days of ceftriaxone.
At seventh day of antibiotherapy, in the blood cultures grew Pasteurella canis sensitive to amoxicillin-clavulanate, cefotaxime and cefoxitin. It was resistant to gentamicin, penicillin and trimethoprim-sulfamethoxazole.
The patient was treated with ceftriaxone for six weeks with a favourable clinical evolution (maintained apyrexia, repeated blood cultures without bacterial growth and laboratory tests without elevation of inflammatory parameters). He made an uneventful recovery and maintained follow-up in Internal Medicine and Cardiology consultation, aiming an elective valve replacement surgery.
Discussion
P. canis invasive infection is a rare entity. After a review of the literature, we found six cases of P. Canis bacteraemia but no description of endocarditis. 2,4,6,7,8
Immunocompromised individuals, namely those with liver disease, hematologic malignancies or solid organ transplantation are particularly at risk for Pasteurella severe infection. 4Pasteurella canis bacteraemia described in the literature were seen in people with underlying diseases namely cirrhosis, chronic lung diseases or in the extreme ages of life.2,6,7 However, Pasteurella infection can also occur in healthy individuals as it was reported by Casallas-Rivera et al.6Soft tissue and wound infections are the most common site for Pasteurella infection followed by the respiratory tract.2Some infections may occur in the absence of animal contact.5 In this case, there was no bite history, only a close contact with a dog. This patient did not have any immune deficiency documented even after an exhaustive investigation and close follow-up.The endocarditis location was also unusual but probably related to the endothelium lesion caused by regurgitant jet due to aortic insufficiency.
The subacute disease evolution associated with the epidemiological context were suggestive of a less common microbiological agent and therefore he was treated empirically with a third-generation cephalosporin (which have a broad spectrum of activity), showing gradual improvement. Usually, Pasteurella is susceptible to Penicillin, amoxicillin-clavulanate, piperacillin, fluoroquinolones, newer generation cephalosporins (ceftriaxone, cefixime), doxycycline and carbapenems.2 Treatment failures were reported with macrolides, oxacillin, first generation cephalosporin and clindamycin.10
In this case, P. Canis was susceptible to 3rd generation cephalosporin. Although guidelines recommend therapy with bactericidal combinations of betalactams and aminoglycosides in non-HACEK gram-negative bacilli endocarditis, patient presented a significant clinical improvement only with ceftriaxone and P. canis was resistant to gentamicin.10Once he was asymptomatic, without cardiovascular symptoms, valve replacement was deferred.
P. canis infection is rare, but can occur in humans with life-threatening manifestations.We emphasize the importance of a detailed clinical history, with a proper epidemiological evaluation (especially for animal exposure). An active investigation to identify less common microorganisms is important so the proper treatment could be established.
Figura I
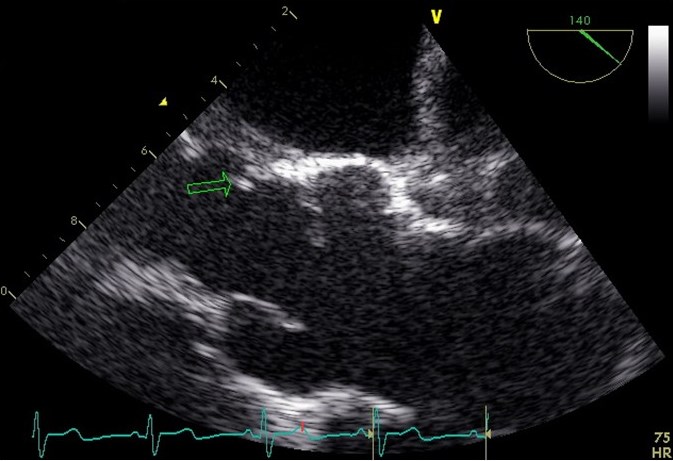
Transesophageal echocardiography: two vegetations in the left ventricular outflow tract
BIBLIOGRAFIA
1 - Pasteurella multocida prosthetic valve endocarditis: case report and review. Clinical infectious diseases. 1997; 25: p. 920-921.
2 - Bhat S, Acharya PR, Biranthabail D, et al. A Case of Lower Respiratory Tract Infection with Canine-associated Pasteurella canis in a Patient with Chronic Obstructive Pulmonary Disease. Journal of Clinical and Diagnostic Research. 2015; 9: p. 3-4.
3 - Albert TJ, Stevens DL. The first case of Pasteurella canis bacteremia: a cirrhotic patient with an open leg wound. Infection. 2010; 38: p. 483–485.
4 - Faceira A, Póvoa S, Souteiro P, et al. Human infection by Pasteurella canis – A case report. Porto Biomed. J. 2017; 2: p. 63-65.
5 - Rivera MC, Martínez AF, Beltrán NP, et al. Septicemia hemorrágica y empiema pleural por Pasteurella canis. Rev Chilena Infectol. 2016; 33: p. 85-88.
6 - Swati N, Dnyaneshwari G, Arvind B. Unusual infection: Pasteurella canis bacteremia in a Child. Global Research Analysis. 2013; 2.
7 - Yefet E, Abozaid S, Nasser W, et al. Unusual infection--Pasteurella canis bacteremia in a child after exposure to rabbit secretions. Harefuah. 2011; 150(1): p. 13-15.
8 - Zurlo, John J. Pasteurella Species. In Bennett JE DRBM. Principles and Practice of Infectious Diseases.: Elsevier Saunders; 2015. p. 2603-2606.
9 - Kaftandzieva A, Peneva M, Petrovska B, et al. Pasteurella Canis as a cause of soft-tissue infection after dog bite: a Case Report. Maced J Med Sci. 2013; 6(1): p. 74-78.
10 - Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis. European Heart Journal. 2015; 36: p. 3075–3123.


