Introduction:
The antiphospholipid syndrome (APS) is a prothrombotic condition, which can affect all segments of the vascular bed.1,2
The diagnosis requires the combination of at least one clinical criteria and at least one laboratorial criteria (updated Sapporo or Sydney Criteria).3 Clinical criteria include venous or arterial thrombosis and/or pregnancy morbidity. The laboratorial criteria refer to antiphospholipid antibodies (lupus anticoagulant, anticardiolipin antibodies and anti-β-2glycoprotein-I antibody). Antibodies must be measured on two or more occasions at least 12 weeks apart. The diagnosis of APS require antibody levels to be persistent and above determined limits. If less than 12 weeks or more than 5 years separate the positive antiphospholipid antibodies test and the clinical event, an APS diagnosis should not be considered.3
Durcan and Petri estimate that the APS prevalence is around 40-50 per 100, 000 persons and the incidence 5 new cases per 100,000 persons/year. 4 APS is more common in women.5
APS can appear as a single disease or be associated with another autoimmune disease, Systemic Lupus Erythematosus being the most frequent.3
The Euro-Phospholipid Project Group studied 1000 patients and found that the most common manifestations are deep vein thrombosis (39%), thrombocytopenia (30%), livedo reticularis (24%), stroke (20%), pulmonary embolism (14%), superficial thrombophlebitis (12%), transient ischemic attack (11%), and hemolytic anemia (10%).5
Budd-Chiari syndrome (BCS), associated with morphological changes resulting from hepatic venous outflow tract obstruction, is a rare manifestation of APS.6 BCS occurs in less than 0.7% of APS cases.5
Case Report:
A male patient, 46 years old, with a previous hospital admission for hypovolemic shock. Lung exam revealed no air flow on the right side and the patient complained of epigastric pain on abdominal palpation.
Chest radiograph and an abdominal ultrasound showed an extensive right pleural effusion. A thoracic drain was placed with output of hematic pleural effusion. (Figure 1) A thoracic computed tomography (TCT) was also performed that confirmed the presence of a right massive hemothorax.
During surgery, it appeared that the massive right hemothorax was secondary to an apparent spontaneous venous rupture of a right diaphragmatic vein. This vein had a distended, aneurysmatic morphology. The vein was lacquered and the patient was admitted in the Intensive Care Unit (ICU).
The patient denied any precipitating factors, including violent physical exertion, alcoholic habits or known vascular malformations. To study this event, the patient repeated TCT and performed a thoracoabdominal magnetic resonance angiography. These imaging studies’ did not show any vascular malformation or portal hypertension.
After 5 days in ICU, the patient continued care in the surgery ward. The patient progressed favorably and after 11 days of hospitalization, he met conditions for hospital discharge.
Four years later, the patient was referred to an Internal Medicine consult for persistent thrombocytopenia, presenting since the previous episode. Blood workup revealed positive lupus anticoagulant and high levels of anti-β-2glycoprotein-I antibody. (Table I) He denied a history of thrombotic events.
During the follow up consult, the patient repeated the analytical study after 12 weeks, which revealed high levels of anti-β-2glycoprotein-I antibody, additionally demonstrating mildly elevated liver enzymes. The remaining blood work, including coagulation evaluation, autoimmunity and assessment of overall thrombotic risk factor, proved negative. (Table I) The echocardiography did not show significant changes.
Due to the elevated liver enzymes, an abdominal ultrasound was performed that did not expose significant changes. An abdominal computed tomography was then requested that presented alterations suggestive of chronic BCS: complete occlusion of the inferior vena cava (IVC) at the diaphragm; caudate lobe hypertrophy with periportal edema; ectasia of the hepatic, port, azygos and hemiazygos veins; esophageal and right diaphragm varices; mild splenomegaly. (Figure 2-4) Hepatic ecodoppler corroborated the hypothesis of BCS. An upper digestive endoscopy was performed, which did not reveal any signs of portal hypertension.
The first imaging studies carried out were reviewed and compared with the current ones. At that time, some of the structural changes suggestive of BCS were already present, but were not valued in that clinical context. This process has become chronic, so currently the imaging studies show alterations suggestive of chronic BCS.
The patient presented with thrombocytopenia, confirmed high levels of anti-β-2glycoprotein-I antibody and a thrombotic event revealed by BCS. BCS was the presenting manifestation of APS.
Presently, the patient is medicated with warfarin (INR 2.0-3.0) and has been referred to a gastroenterology consultation.
Discussion:
BCS, an uncommon disease, is characterized by structural and functional abnormalities of the liver caused by obstruction to the outflow of hepatic venous blood.7, 8 The obstruction of IVC is more common in Asian patients.9
Clinically, BCS has several manifestations, which may range from asymptomatic to fulminant liver failure.10 Abdominal pain, hepatomegaly and ascites constitute the classic triad of BCS.10
There are several causes of BCS, classified as primary or secondary. BCS is primary if there is a primarily venous process and secondary if the obstruction is related to a lesion originating outside the veins.9
The major causes of primary BCS are myeloproliferative disorders (40-50%), hyperhomocysteinemia (37%), factor V Leiden mutation (6-32%), protein C deficiency (10-30%), antiphospholipid syndrome (4-25%), protein S deficiency (7-20%) and recent pregnancy (6-12%). Recent oral contraceptive use contributes in 6-60% of the cases.9
The relationship between APS and BCS was first documented in 1984.11 Their pathogenic association is controversial.10 Aggarwal et al suggests that structural liver abnormalities of BCS, could be involved in the production of anticardiolipin antibodies, but this might be secondary to the liver damage only.12 Espinosa et al documented cases were APS antibodies were presented before BCS.10
Thrombocytopenia is very common in APS (30%), which was the first expression of APS that the authors found in this patient.5 The patient met the analytical criteria for APS. After a careful review of the patient´s medical history, the authors’ hypothesis is that the patient presented with a spontaneous hemothorax secondary to structural and functional changes caused by BCS. Therefore, BCS was the probable initial manifestation of APS.
The main causes of spontaneous hemothorax are coagulopathy (congenital disease/ drug related), vascular (arteriovenous malformations/aneurysms/connective tissue disease) and neoplasia (vascular tumors/hepatocellular carcinoma).
The authors believe that thrombosis and complete occlusion of the IVC was a gradual process. In a first phase, thrombosis may not have been complete, allowing time for the development of compensatory mechanisms with the development of collateral circulation. During this time, clinical manifestations would be absent. With eventual complete occlusion of IVC, blood circulation in and around the liver underwent structural changes. By this time, the collateral venous circulation was well established by the ascending lumbar vein, azygos and hemiazygos veins or paravertebral veins. Increased pressure in the veins resulted in retrograde flow in the azygos tributary veins.14 This hypothesis is corroborated by Okuda.14 This process was responsible for the venous rupture of the right diaphragmatic vein, leading to the spontaneous hemothorax. Bleeding complications in patients with APS are rare.11
In the first stage, the thrombus could be organized into a membrane, and in the chronic stage shrunk, with concomitant narrowing of the IVC lumen.14
The gradual occlusion of the IVC leads to high intrahepatic venous pressure, which will eventually be complicated by hepatic cirrhosis.14
The authors suspect that the first imaging studies did not reveal any vascular change because of the low diameter of right diaphragmatic vein, massive hemothorax and hypovolemic shock with compensatory vasoconstriction. The authors believe that in this episode, if it was done a thoracic and abdominal imaging study, few months later of the acute event, the diagnosis of BCS could be made earlier.
In a series of 43 patients with BCS and APS, there were 67% females with a mean age of 30.8±12.3 years.10 A total of 74% of the patients had APS with no other associated disease. In 65% of the cases, BCS was the first clinical manifestation of APS. The most common presenting symptom was abdominal pain (56%). Obstruction of hepatic veins was found in 77% of the patients. Anticoagulation was the most frequent treatment (84% of the patients), followed by steroids (37%) and aspirin (11%).
Despite treatment, APS is associated with significant morbidity and mortality.15 Long-term prognosis in APS is most influenced by the risk of recurrent thrombosis.2 The cornerstone of prevention and treatment is anticoagulation ad eternum.16 Bleeding manifestations and adverse effects can complicate and contribute to increased morbidity.1
There is insufficient evidence to make recommendations about newer oral anticoagulant therapies in APS patients. Some research shows that Dabigatran and Rivaroxaban have been used with success.17,18,19
The initial treatment of BCS is anticoagulation and treatment of the underlying condition.9 If initial treatments fail, other options include transjugular intrahepatic portosystemic shunt and liver transplant.20
Asymptomatic BCS, associated with extensive collateral vasculature, has a favorable prognosis at 3 years, and surgical therapy may not be required.21
The present case is the first Portuguese report of the rare association of BCS and APS (with no other autoimmune disease). In addition, there are no reports in the literature of hemothorax due to IVC thrombosis.
This case is very interesting, includes a unique association: hemothorax, BCS and APS. All these clinical considerations were important for the correct treatment, improving the prognosis.
Figura I
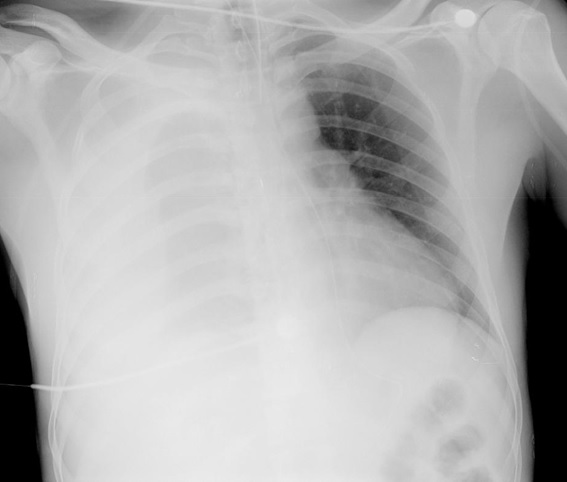
Chest radiograph of the patient at admission in the first episode.
Figura II
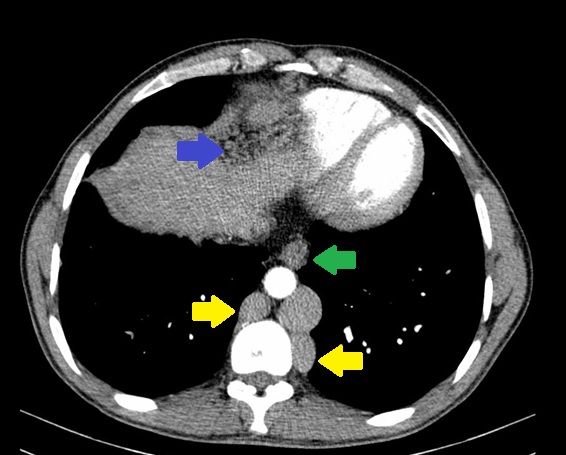
Abdominal computed tomography: ectasia of azygos and hemiazygos veins (yellow), esophageal (green) and right diaphragm varices (blue).
Figura III
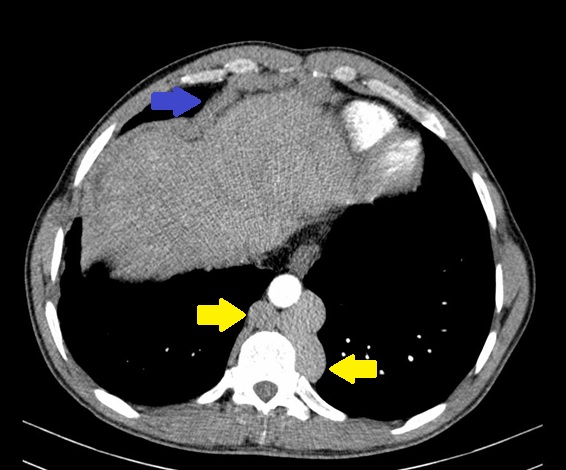
Abdominal computed tomography: ectasia of azygos and hemiazygos veins (yellow), right diaphragm varices (blue).
Figura IV
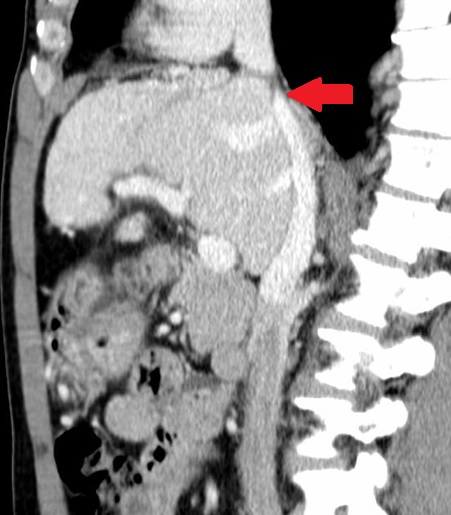
Abdominal computed tomography: occlusion of the inferior vena cava at the diaphragm (red).
Figura V
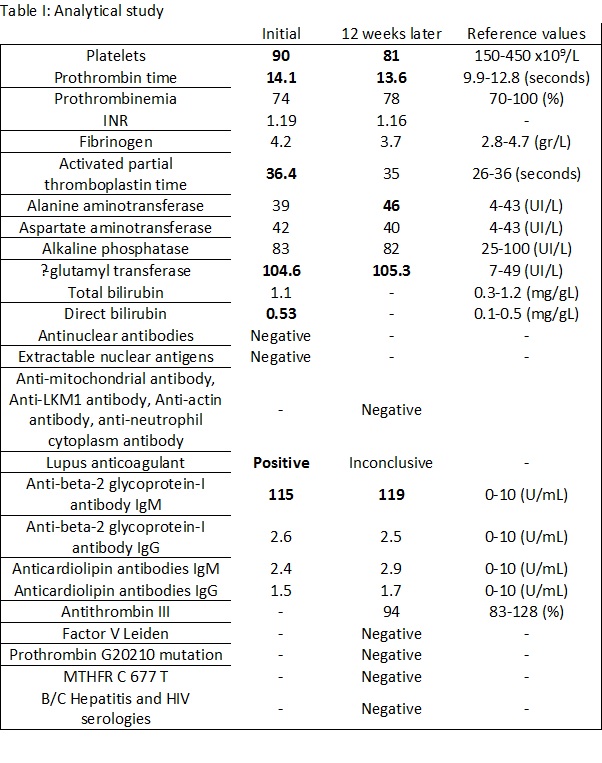
Table I: Analytical study
BIBLIOGRAFIA
(1): Lim W. Antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program. 2013; 2013: 675-680.
(2): Khamashta MA, Bertolaccini ML, Hughes GRV. Antiphospholipid (Hughes) Syndrome. Autoimmunity. 2004; 37: 309-312.
(3): Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). Journal of Thrombosis and Haemostasis. 2006; 4: 295–306.
(4): Durcan L, Petri M. Epidemiology of the Antiphospholipid Syndrome. In: Cervera R, Espinosa, Khamashta MA. Antiphospholipid syndrome in systemic autoimmune diseases. Amsterdam: Elsevier; 2016. 17–30.
(5): Cervera R, Piette JC, Font J, et al. Antiphospholipid Syndrome Clinical and Immunologic Manifestations and Patterns of Disease Expression in a Cohort of 1,000 Patients. Arthritis & Rheumatism. 2002; 46: 1019-1027.
(6): Uthman I, Khamashta M. The abdominal manifestations of the antiphospholipid syndrome. Rheumatology. 2007; 46: 1641-1647.
(7): Enestvedt CK, Orloff SL. Budd-Chiari syndrome and veno-occlusive disease. In: Jarnagin W. Blumgart’s Surgery of the Liver, Biliary Tract and Pancreas. Philadelphia: Elsevier; 2016. 1248-1271.
(8): Dilawari JB, Bambery P, Chawla Y, et al. Hepatic Outflow Obstruction (Budd-Chiari Syndrome) Experience with 177 Patients and a Review of the Literature. Medicine. 1994; 73: 21-36.
(9): Deleve LD, Valla DC, Garcia-Tsao G. Vascular Disorders of the Liver. Hepatology. 2009; 49: 1729-1764.
(10): Espinosa G, Font J, García-Pagan JG, et al. Budd-Chiari Syndrome Secondary to Antiphospholipid Syndrome Clinical and Immunologic Characteristics of 43 Patients. Medicine. 2001; 80: 345-354.
(11): Pomeroy C, Knodell RG, Swaim WR, Arneson P, Mahowald ML. Budd-Chiari Syndrome in a Patient With the Lupus Anticoagulant. Gastroesterology. 1984; 86: 158-161.
(12): Aggarwal R, Ravishankar B, Misra R, Aggarwal A, Dwivedi S, Naik SR. Significance of Elevated IgG Anticardiolipin Antibody Levels in Patients With Budd-Chiari Syndrome. The American Journal of Gastroenterology. 1998; 93: 954-957.
(13): Patrini D, Panagiotopoulos N, Pararajasingham J, Gvinianidze L, Iqbal Y, Lawrence D. Etiology and management of spontaneous haemothorax. Journal of Thoracic Disease. 2015; 7(3): 520-526.
(14): Okuda K. Inferior Vena Cava Thrombosis at Its Hepatic Portion (Obliterative Hepatocavopathy). Seminars in Liver Disease. 2002; 22: 15-26.
(15): Cervera R, Serrano R, Pons-Estel GJ, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2014; 0: 1-8.
(16): Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: Report of a Task Force at the 13th International Congress on Antiphospholipid Antibodies. Lupus. 2011; 20: 2016-2018.
(17): Andrade D, Cervera R, Cohen H, et al. 15th International Congress on Antiphospholipid Antibodies Task Force on Antiphospholipid Syndrome Treatment Trends Report. In Erkan D, Lockshin MD. Antiphospholipid Syndrome: Current Research Highlights and Clinical Insights. Cham: Springer; 2017. 317-338.
(18): Reshetnyak TM, Seredavkina NV, Satybaldyeva MA, Nasonov EL, Reshetnyak VI. Liver transplantation in a patient with primary antiphospholipid syndrome and Budd-Chiari syndrome. World J Hepatol. 2015; 7: 2229-2236.
(19): Cohen H, Hunt BJ, Efhymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016; 3: e426-436.
(20): Hamilton JP. The Management of Budd-Chiari Syndrome. In: Cameron J, Cameron A. Current Surgical Therapy. Philadelphia: Elsevier; 2017. 421-425.
(21): Hadengue A, Poliquin M, Vilgrain V, Belghiti J, Erlinger CDS, Benhamou JP. The Changing Scene of Hepatic Vein Thrombosis: Recognition of Asymptomatic Cases. Gastroenterology. 1994; 106: 1042-1047.






