Introduction: Splenic cysts are rare entities with around 800 cases described in the literature.1Most of these cysts are diagnosed accidentally during the workup for general complaints or abdominal imaging. Due to their slow growth the symptoms normally result from a mass effect on another organ, such as nausea, vomiting and flatulence, and in some instances may cause pleuritic pain, dyspnea and persistent cough.Case ReportWe present the case of a 32 year-old man natural from the north of Portugal. He had a personal history of Ankylosing Spondylitis but was in remission without the need for any medication and was referenced by his general practitioner because of post-prandial fullness and left upper quadrant pain with the finding on abdominal ultrasound of a splenic cyst of 7.5x8 cm with multiple septa suggestive of a hydatic cyst. The patient had been told four years before that he had a cyst of the spleen. From an epidemiologic perspective he hadn´t taken any recent trips out of Western Europe but did have dogs in his home town which he cared for frequently. On examination he had no remarkable findings, namely no abdominal pain or an enlarged liver or spleen on palpation, and no cutaneous lesions. In order to confirm this finding his GP had requested an MRI which showed a cyst, on the upper pole of the spleen, of 6.5x7.5cm with pure content and a thin septation, which was reported as not suggestive of an hydatic cyst. A laboratory workup was conducted with serology for Equinococcus granulosus by immunodiffusion and agglutination, which were negative, but due to a high suspicion they were repeated in a different institution by an ELISA method that was also negative.Due to the growth rhythm (about 1 cm per year) comparing the ultrasound from 4 years prior and the splenomegaly, it was discussed both with radiologists and surgeons and was decided that a splenectomy was the best approach. The patient got the necessary immunizations, Haemophilus influenza B, both vaccines for Streptococcus pneumoniae, Prevnar® (PCV 13) and Pneumo 23® (PPSV23), with a 8 week interval between them and Meningococcus (for serotype C due to the fact that he didn´t travel to endemic countries2) that he completed 6 weeks before surgery. He was given flu vaccine. Prior to the surgery he started a 4 week course of albendazole adjusted to his weight (400 mg bid) up until the surgery. The surgery was performed laparoscopically and without complications (Figure 1). The spleen measured 16x8,5 cm and had multiple thin walled cysts, the biggest with 6,5 cm (Figure 2).DiscussionSplenic cysts were first classified by Martin:3Type 1 – primary or true cysts, also known as epithelial or epidermoid, with epithelial capsule, which can be either parasitic (most of them caused by Echinococcus granulosus) or not. The latter ones may be congenital, vascular or neoplastic; Type 2 – secondary or pseudocysts, which have no capsule and are usually post-traumatic or inflammatory/degenerative. Despite this difference radiologically it is usually very difficult to distinguish primary from secondary cysts. It is the histology of the cyst wall that allows the distinction between true and false cysts.1The presence of an epithelial lining in type 1 cysts denotes a congenital origin. They can further be subclassified as parasitic (most of them caused by Echinococcus granulosus) and non-parasitic. The non-parasitic cysts can be subdivided in congenital or neoplastic. The congenital cysts may be epidermoid – resulting from inclusion of adjacent epithelial cells or invagination of the mesothelium; dermoid – considered to be terratomas because its structures originated from all three germ layers; and endodermoid - which are not true cysts as they are composed of vascular structures and should be classified as lymphangiomas or hemangiomas. Finally there may be the neoplastic cysts (type 1, non-parasitic) of the spleen4.
Type 1 non-parasitic congenital cyst is the most common type found in young adults, most of which are epidermoid4. The differential diagnosis includes splenomegaly, as a manifestation of a systemic disorder (e.g. infections, hematological diseases, primary tumors) as well as cysts, abscesses and tumors of surrounding organs, like pancreatic pseudocysts, that protrude to the spleen4. The workup of these lesions is done with ultrasound and CT scan. Sonography has an important role in the initial approach to a splenic cyst which typically appears round, homogeneous and with an anechoic area with a smooth, thin wall4. In the presence of daughter cysts and various membranes in the cyst a parasitic etiology should be considered5. CT scan can help determine the morphology of cyst, the nature of fluid, the exact location and its relationship with adjacent structures6.
When there is a suspicion of a parasitic cyst the use of serological studies complement the imaging studies - tests such as ELISA, immunoelectrophoresis and indirect hemagglutination test (HIA) – which have both a high sensitivity and specificity8. When the serologies are negative and the imaging studies inconclusive, an MRI can be helpful to differentiate parasitic cysts from other types as these latter typically present a low intensity rim characteristic of hydatidosis.5This image modality is also useful for surgery planning to better understand cyst relation to other organs. There are no guidelines for definite management. The traditional approach to splenic cysts has been the surgical removal of the spleen however, there has recently been a shift towards the preservation of this organ due to its role in the immune system. Other options include percutaneous drainage, partial cystectomy (fenestration) of the cyst or marsupialization of superficial cysts, partial splenectomy.2,5Although an approach where the spleen is preserved should be the main goal special occasions warrant a total splenectomy, namely, polycystic cases, cysts of large dimensions, hilar cysts, intraparenchymal cysts, inaccessible cysts and uncontrollable massive bleeding. Postsplenectomy sepsis is the most feared complication and the preservation of at least 25% of splenic mass gives immunologic protection. Before the removal of the cysts the patient should be immunized against capsulated organisms namely Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenza type B. The regimen and timing of vaccination depends on whether the patient has been vaccinated before undergoing splenectomy.9The recommended immunization for patients not previously vaccinated (the case of our patient) for prevention of of pneumococcal infection is the administration of PCV13 followed 8 weeks later by PPSV23. Other capsulated organs for which vaccination should be given is Haemophilus influenza type B as well as Neisseria meningitidis. All patients should be vaccinated for N. meningitidis serotype C and those who travel to endemic areas should also get immunization for serotype B2. There is insufficient data on the effectiveness or indications and appropriate duration of prophylatic antibiotic therapy in asplenic patients given the routine use of PCV 13 and pneumococcal antimicrobial resistance.5When a hydatic etiology is suspected a prophylactic course before the cyst removal should be undertaken in order to decrease the risk of secondary echinococcus as well as anaphylaxis during surgery if there should be a cyst rupture. Peri-interventional treatment with benzimidazoles is highly recommended for at least for 1 month (albendazole) prior to surgery and the WHO manual10suggests adding praziquantel 2 weeks prior to surgery to increase the number of patients with nonviable parasites as compared to monotherapy with albendazole. However, further studies are needed for evaluating the efficacy of the combined treatment.9This is done with albendazole 10-15 mg/kg/day (maximum dose 800 mg) twice daily. Portugal is one of the regions of the world with the highest prevalence of hydatidosis thus it is one of the differential diagnosis in the study of the splenic cyst. The Echinococcus granulosus is the only species which is indigenous to Portugal where the dog is the final host.10In our case it wasn´t possible to obtain a definite diagnosis with the clinical history or the imaging exams and thus surgery was the only option. Due to its size and location the surgical team decided to proceed with a total splenectomy. This decision was based on the information available in the literature, although the evidence for a cutoff limit isn’t robust4, the generally accepted size is > 5 cm in diameter. It is considered that above this limit there is a higher risk of rupture and thus having a former indication for surgery regardless of its etiology. Since it wasn´t possible to exclude a parasitic cause, and being that Portugal is endemic to hydatidosis, our patient underwent a month of prophylaxis with albendazole in order to prevent dissemination of the parasitic forms during surgery and consequently secondary hydatidosis. He had a weekly check of blood count and transaminases until the surgery without changes.The epidermoid cyst our patient had is the most common of the type 1 non-parasitic cysts (90%)11. It is thought to result from the invagination of the peritoneal mesothelium of the splenic capsule followed by proliferation and scamous metaplasia.12Conclusion:The case we present is a rare case of a splenic cyst which entails a thorough differential diagnosis. It has been 2 years since the surgery and the patient has resumed his normal work and personal life without limitations or infections.Despite the advances in medical technology in the presence of a splenic cyst a hydatid etiology should always be suspected in endemic areas.
Figura I
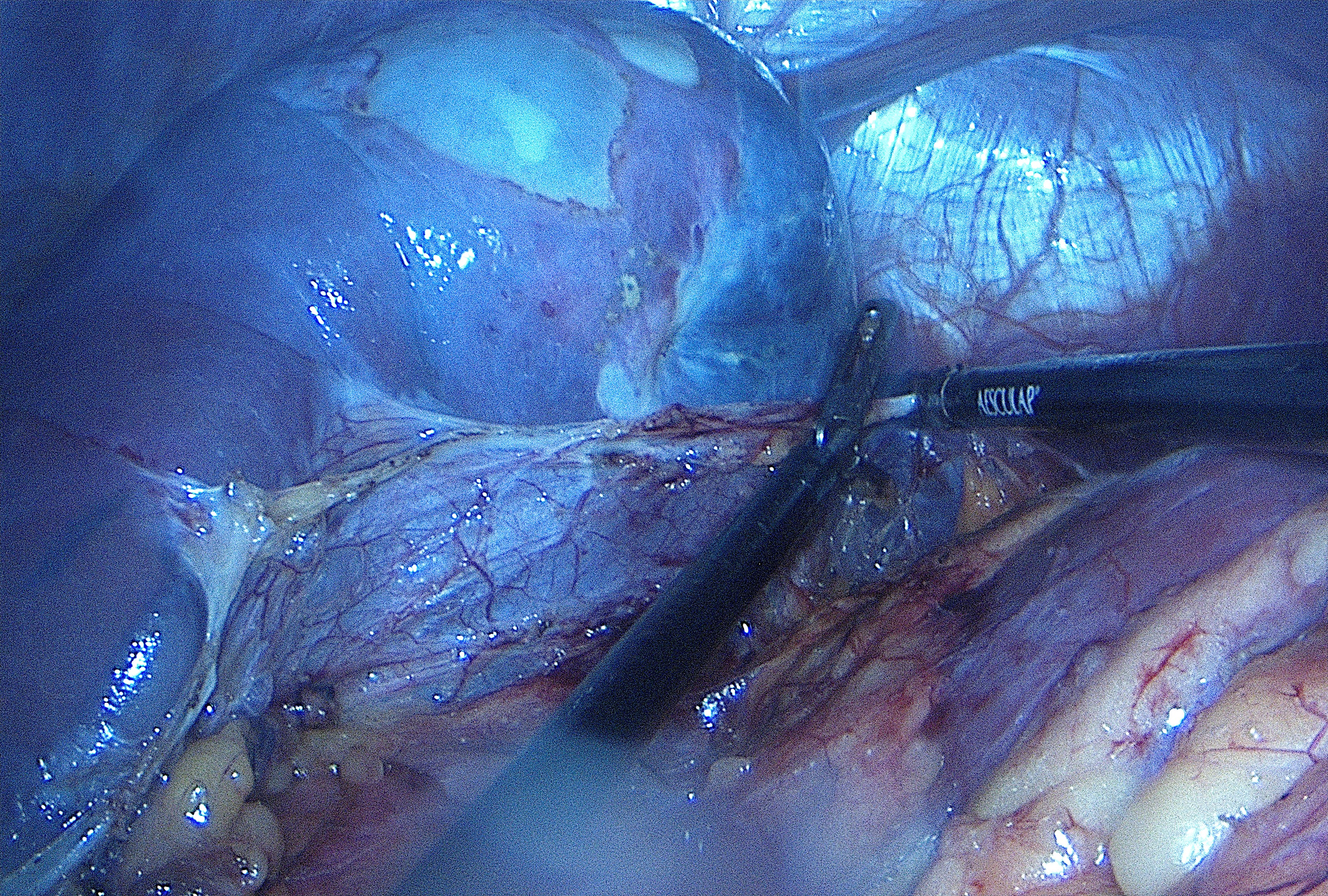
Intraoperative image of the patients´ spleen with some superficial cysts visible.
Figura II
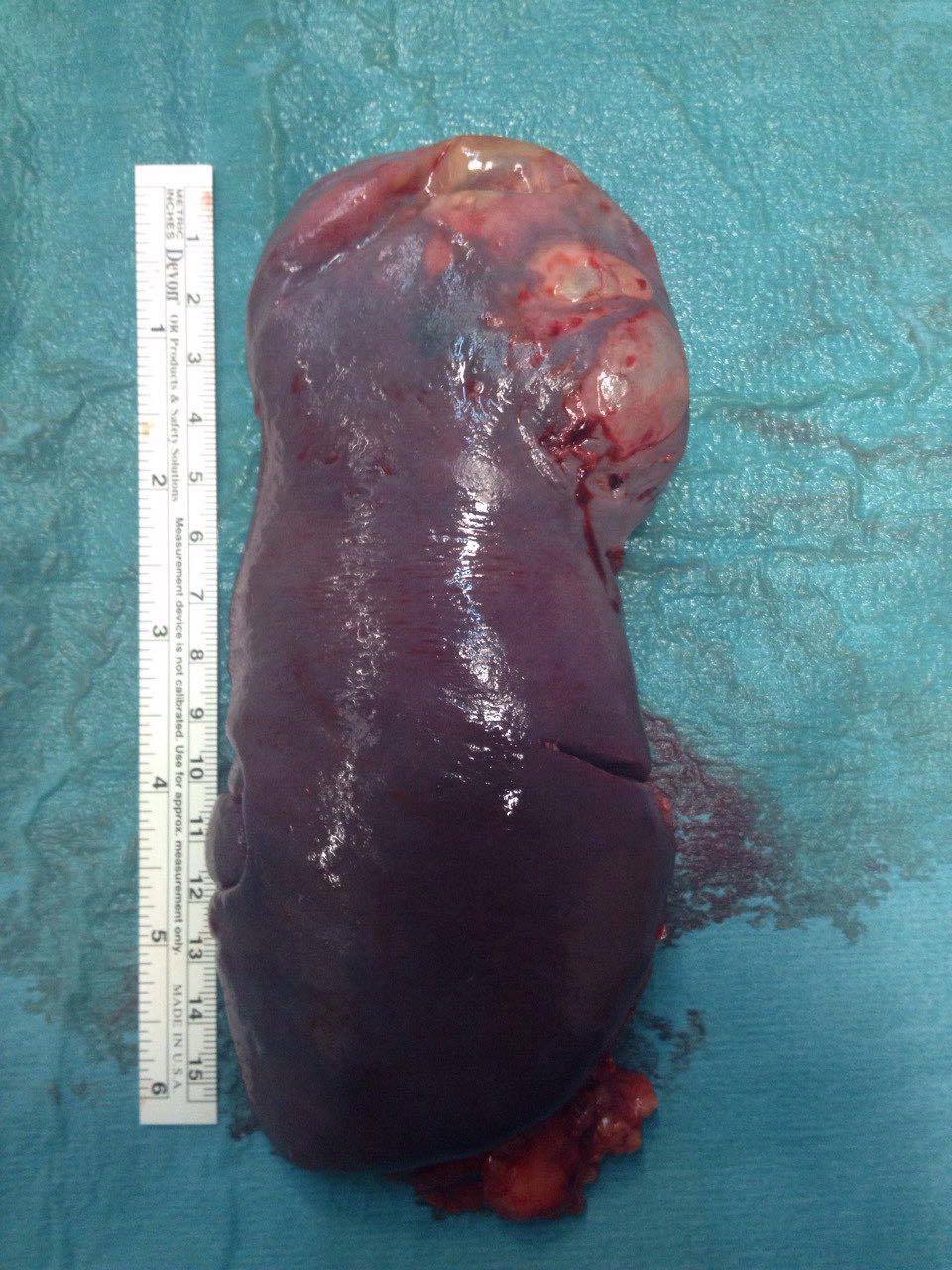
Image of the spleen after being removed.
BIBLIOGRAFIA
1. MEENU, G. et al; “Large splenic epithelial cyst – A rare entity presenting as hydatid cyst”; Human Pathology: Case Reports 10 (2017) 96-97;
2. DUARTE, L., et al; “Prevention of Post-splenectomy Sepsis: creation of a vaccination and patient education guideline” Revista Portuguesa de Cirurgia (2014) (31): 9-18;
3. SCHLITTLER, L. A; DALLAGASPERINA, V. W.; “Non-parasitic splenic cysts”; Rev. Col. Bras. Cir. 2010; 37(6): 442-446;
4. HANSEN, M. B.et al MOLLER, A. C.; “Splenic Cysts”; Surg. Laparosc Endosc Percuntan Tech, volume 14, Number 6, December 2004;
5. RASHEED, K., et al; “Never underestimate simple splenic cyst”; Indian Journal of Medical Sciences, Vol. 66, No. 3 and 4, March and April 2012;
6. INGLE, S. B., et al; “Epithelial cysts of the spleen: A minireview” World J Gastroenterol. 2014 Oct 14; 20(38): 13899–13903.
7. GALYFOS, G. et al; “Oversized pseudocysts of the spleen: Report of two cases Optimal management of oversized pseudycsts of the spleen” International Journal of Surgery Case Reports 5 (2014) 104-107;
8. TAMER, G. S.; et al; “Evaluation of Immunochromatographic Test for the Detection of Antibodies against Echinococcosis granulosus”; Med Sci Monit, 2015; 21: 1219-1222;
9. GOLMOHAMMADZADEH, H.; et al; “Splenic cysts: Analysis of 16 cases” Caspian J. Intern Med 2016; 7 (3): 217-221;
10. ECKERT, J., et al; “WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern”; ©World Organization for Animal Health (Office International des Epizooties) and World Health Organization, 2001;
11. RUBIN, L. G., M.D., SCHAFFNER, W., M.D.;“Care of the asplenic patient”; N Engl J Med 2014;371:349-56.
12. KARFIS, E. A., et al; “Surgical Management of Nonparasitic Splenic Cysts” JSLS. 2009 Apr-Jun; 13(2): 207–212.



