Background
Infective endocarditis (IE) is a systemic life-threatening disease mainly affecting patients with heart valve disease, prosthetic valve, intracardiac devices and intravenous drug abusers. Clinical findings, echocardiography, and blood cultures are the cornerstone of IE diagnostics1,2. Abiotrophia defectiva or nutritionally variant strepcocci (NVS), was first described in 1961 by Frenkel and Hirsch in a case of sub-acute infectious endocarditis. The nomenclature of this bacteria changed in the subsequent years being now classified as the only specie in this genus1,3.
A. defectiva has been described as non-motile, gram-positive cocci in chains that are catalase negative, pyridoxine dependent and exhibit satellitism4,5. It is usually detected with automated blood culture instruments, but it doesn’t grow well on standard, unsupplemented solid media, as it requires pyridoxal or cysteine for growth. This can be accomplished by co-cultivation with staphylococci, by the use of pyridoxal impregnated disks placed on the surface of standard blood agar plates or by the use of specially supplemented or enriched media3,6,7.
A. defectiva is usually found in the oral cavity, as well as, the oropharyngeal, digestive and genitourinary tracts. Endocarditis due to A. defectiva is rare but accounts for up to 6% of streptococcal endocarditis and some cases of hemoculture negative endocarditis3,8,9.
We report a case of infective endocarditis (IE), caused by A. defectiva in an immunocompetent patient, with previous history of heart valve disease and recent dental procedure.
Case report
A 43-year old male with mitral valve prolapse and mild mitral regurgitation (MR) was admitted to our hospital with fever, asthenia, anorexia and weight loss (of about 10% of his body weight) persisting for 4 weeks. Three months before admission he has been submitted to a dental procedure.
On admission, he was febrile (tympanic temperature 38,2ºC) but hemodynamically stable. The examination showed a grade III/VI systolic murmur in the mitral area. No petechiae, splinter haemorrhages, Janeway’s lesions or Osler’s nodes were observed. Fundoscopic examination was normal. Laboratory studies revealed normocytic anaemia (Hb 8.9g/dL), with a leucocyte count of 11980/mL (neutrophil 89%), C-reactive protein of 83 mg/L, erythrocyte sedimentation rate of 101 mm in the first hour and normal ferritin. Sequential transthoracic and transesophageal echocardiograms showed left atrial enlargement, preserved global ventricular function and a vegetation attached to the ventricular surface of the mitral valve (Figure 1) and severe MR (Figure 2).
Two sets of blood cultures were obtained (aerobic and anaerobic culture). Culture of blood from peripheral vein was performed with a BACTEC system with BD BACTEC™ blood culture media. Blood cultures were positive after a 24-hour incubation period. Gram-stained smear showed pleomorphic gram positive cocci. Subsequent culture revealed small, grey, non-hemolytic, catalase negative colonies that only grew on chocolate agar. A.
defectivawas identified with low discrimination using the Vitek 2 system and API Rapid ID 20 Strep (BioMérieux). This identification was confirmed by matrix-assisted laser desorption/ionization time-of-flight mass-spectrometry (MALDI-TOF MS). As there was no database for MIC calculation for A.
defectivain Vitek 2 identification system, anti-microbial susceptibility was determined by E-test method and interpreted according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) PK-PD (non-species related) breakpoints. The isolate was subsequently found to be susceptible to penicillin, third generation cephalosporins and vancomycin. The patient was treated with amoxicillin and clavulanic acid 2,2g tid for 6 weeks and gentamicin 160 mg tid for 2 weeks. A marked improvement of clinical signs and symptoms and fall in the inflammatory markers was observed.
Despite antibiotic treatment adapted to the antimicrobial susceptibility pattern, severe MR remained present and mitral valve replacement with a mechanical valve was performed with complete disease resolution. No valve abcess was reported and valve culture was negative. After 60 days the patient was discharged home and was doing well at the last follow-up 12 months after surgery.
Discussion
A. defectiva is usually part of the normal oral microbiota and usually enters the bloodstream through the mouth, teeth or throat3,9,10. Until 2012, nearly 125 cases of A.defectiva endocarditis were described in the literature3,5,9-12. Unlike the genera Granulicatella which is observed in neutropenic patients, endocarditis caused by the genus Abiotrophia can occur in immnunocompetent environment8. Often there is an history of dental manipulation in the preceding months or a history of dental caries10. Our patient had no immunodeficiency status and presented with lengthened constitutional symptoms and a recent dental procedure, making the oral cavity the port of entry. A.defectiva affects diseased valves in 90% of cases and is involved in systemic embolism and valve destruction despite the antibiotics11.
Because it’s a fastidious organism, A.defectiva is difficult to identify with the standard blood agar and it is likely that most cases will be misdiagnosed as negative culture IE8,9,12. A. defectiva has many bacterial characteristics: vitamin B6 and L-cysteine are required for growth, thus this bacteria cannot grow in culture plates without these nutrients6,7,12. It grows more slowly than other streptococci, so extended incubation may be require7. Prompt diagnosis is not always possible for two main reasons: first, the organism does not grow well on standard blood agar without supplementation; second, once colonies grow, they are still difficult to identify with the limited microbiological diagnostic technology available in most microbiology laboratories13,14. In this case, the definitive diagnosis was arduous. The Vitek 2 system (bioMerieux) was only able to identify A. defectiva with low discrimination. A matrix-assisted laser desorption ionisation time of flight mass spectrometry (MALDI-TOF-MS) could greatly reduce time to identification. It is difficult to draw the profile of sensitivity to the antibiotics for A. defectiva. The results obtained in vitro are not correlated to those in vivo because of the rarity of the isolation of A.defectiva and the absence of standardized data for the study of the antibiogram13,14.
Although an uncommon cause of endocarditis, endocarditis caused by NVS carries greater morbidity and mortality than endocarditis caused by streptococci10,15. Despite in vitro sensitivity to several antibiotics, A. defectiva still causes significant complications. The main complications of A.defectiva endocarditis reported in the literature are acute heart falure and septic emboli increasing the need of surgical interventions4,5. Historically, A. defectiva has declared itself more difficult to treat than Streptococcus. A high resistant rate to betalactams and macrolides has been found in several reports10,14. However, The American Heart Association recommends that the treatment regime used is the same as that used for Enterococcus endocarditis. Typically, this consists of ampicillin or penicillin G in addition to gentamicin for a 4-6 week period. Alternatively, vancomycin plus gentamicin for 6-week period can be used1. Our patient had previous valvular disease and developed MR worsening despite medical treatment with agent-directed antibiotics. As described in literature, valve replacement was needed to recover valve function.
Conclusion
IE due to A.defectiva is a rare entity. The identification of such organism is difficult but vital, as it can be the origin of a therapeutic failure, severe systemic complications and need of valve surgery. Clinical microbiology laboratories must be alert to the fact that pleomorphic, variable Gram-staining organisms that selectively grow only from enriched (i.e. chocolate) media are key diagnostic indicators of A. defectiva. This case highlights the relevance of a correct diagnosis of an uncommon pathogen that can be misdiagnosed as culture negative endocarditis. A prompt and aggressive treatment and a very strict surveillance of these patients is necessary to improve outcomes.
Figura I
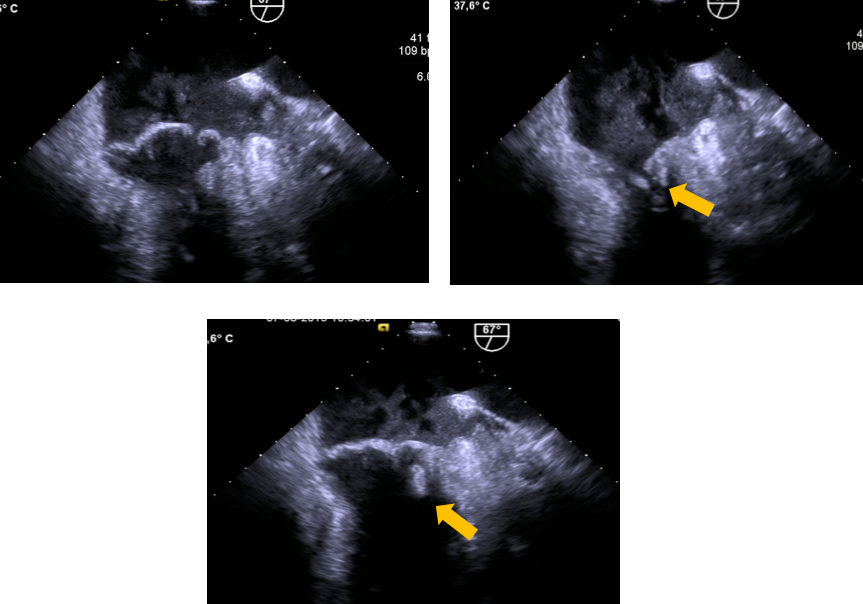
Transoesophageal echocardiography (TEE) of a mitral valve prolapse. These TEE-based 60° views shows greater than 2-mm superior displacement of the mitral leaflets into the left atrium during systole, with a posterior leaflet thickness of at least 5 mm and a vegetation on the ventricular surface of posterior leaflet scallop P1 (antero-lateral) (yellow arrows)
Figura II
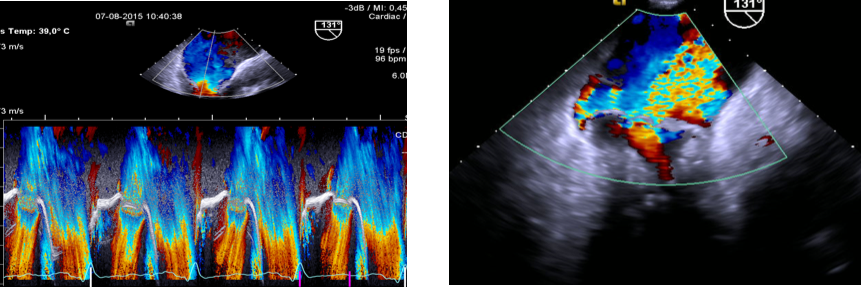
Presence of severe mitral regurgitation (indicated by high-velocity eccentric jet during late systole). In general, the detection of a large eccentric jet adhering, swirling and reaching the posterior wall of the left atrium is in favour of significant mitral regurgitation.
BIBLIOGRAFIA
1. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, et al. Infective endocarditis diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: Endorsed by the Infectious Diseases Society of America. Circulation. 2005; 111:e394–434.
2. Zamorano J, Perez de Isla L, Moura L, Almería C, Rodrigo JL, Aubele A, et al. Impact of Echocardiography in the Short- and Long-term Prognosis of Patients with Infective Endocarditis and Negative Blood Cultures. J Heart Valve Dis. 2004; 13: 997-1004
3. Collins MD, Lawson PA. The genus Abiotrophia is not monophyletic: proposal of Granulicatella gen. nov., Granulicatella adiacens comb. nov., Granulicatella elegans comb. nov. and Granulicatella balaenopterae comb. nov. Int J Syst Evol Microbiol. 2000; 50: 365-369.
4. Christensen JJ, Facklam RR. Granulicatella and Abiotrophia species from human clinical specimens. J Clin Microbiol 2001;39:3520–3.
5. Ramos J, Dos Santos L, Vidal LM, Pereira PM, Salgado AA, Fortes CQ, et al. A case report and literature overview: Abiotrophia defectiva aortic valve endocarditis in developing countries. Infection. 2014; 42:57984.
6. Kawamura Y, Hou XG, Sultana F, Liu S, Yamamoto H, Ezati T. Transfer of Streptococcus adjacens and Streptococcus defectivus to Abiotrophia gen. nov. as Abiotrophia adiacens comb. Nov and Abiotrophia defectiva comb. Nov., respectively. Int J Syst Bacteriol. 1995; 45:798-803.
7. Kirn TJ, Weinstein MP. Update on blood cultures: how to obtain, process, report and interpret. Rev Clinical Microbiology and Infection. 2013; 19:513-520.
8. Senn L, Entenza JM, Greub G, Jaton K, Wenger A, Bille J, et al. Bloodstream and endovascular infections due to Abiotrophia defectiva and Granulicatella species. BMC Infect Dis. 2006; 6:9.
9. Giuliano S, Caccese R, Carfagna P, Vena A, Falcone M, Venditti M. Endocarditis caused by nutritionally variant streptococci: a case report and literature review. Infez Med. 2012; 20:67–74.
10. Carleo MA, Del Giudice A, Viglietti R, Rosario P, Esposito V. Aortic valve endocarditis caused by Abiotrophia defectiva: case report and literature overview. In vivo. 2015; 29:515-518.
11. Roberts RB, Krieger AG, Schiller NL, Gross KC. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis 1979;1:955-66.
12. Malaisri C, Sungkanuparph. Infectious endocarditis cause by Abiotrophia defective. J Infect Dis Antimicrob Agents. 2012; 29:21-25.
13. Pinkney JA, Nagassar RP, Roye-Green KJ, Ferguson T. Abiotrophia defectiva endocarditis. BMJ Case Rep. 2014: 207361.
14. Kadri Y, Hassine M, Echahed C, Chaouch C, Boussaada M, Ben Habdallah H, et al. Infective Endocarditis Caused byAbiotrophia Defectiva: A Case Report. American Journal of Medical Case Reports. 2015; 3: 187-190.
15. Stein DS, Nelson KE. Endocarditis due to nutrionally deficient streptococci: therapeutic dilemma. Rev Infect Dis. 1987; 9:908-916.



