INTRODUCTION
Anterior mediastinal masses are relatively uncommon, nonetheless comprise clinically important diseases, like thymic neoplasia (35%), lymphoma (25%), thyroid and other endocrine tumors (15%), benign teratoma (10%), malignant germ cell tumors (10%) and benign thymic lesions (5%).1
Rheumatoid arthritis (RA) is a condition with a 10% increased risk in overall malignancy compared with general population,2 particularly lymphomas (from which Hodgkin disease is the most frequent) ranging from almost a doubling to a 12-fold increase.2-4 Given the autoimmune nature of RA, although rarely described, thymic hyperplasia (TH), should also be considered, especially in young and asymptomatic patients.1, 5, 6
Herein we report a case of a young patient with RA and TH detected on persistent relative lymphocytosis work-up.
CASE REPORT
We are presenting a 28-year-old male patient with five years history of RA, under methotrexate 10 mg/week, in remission according to disease activity score-28 (DAS28). In routine monitoring, he reported, in the previous month, flu-like symptoms (asthenia, myalgias, sudoresis, without fever) like his child, resolved within one week. He denied cough, loss of weight, abdominal pain, pruritus or other symptoms since then. Physical examination was unremarkable. Highlighted on laboratory tests (Table 1) was relative lymphocytosis (3 100 x109/L), without leukocytosis or neutrophilia, peripheral blood smear with reactive lymphocytes, neutrophils with toxic granulations and slightly elevated aminotransferases (aspartate transaminase 35 U/L and alanine transaminase 67 U/L). Relative lymphocytosis and hepatitis were interpreted as a probable viral infection. However, since liver toxicity due to methotrexate could not be ruled out, it´s dose was reduced to 7,5 mg/week. Four months later, the symptoms resolved, the physical examination was normal (without palpable liver or lymphadenopathies) and the transaminases normalized. However, relative lymphocytosis (2 900 x109/L) remained (Table 1).
Additional tests were performed, including lactate dehydrogenase which was normal, cytomegalovirus (CMV) serologies suggesting recent infection, Epstein-Barr virus and toxoplasma serologies suggested previous infection, screening for syphilis (with venereal disease research laboratory) and human immunodeficiency virus which were negative. Given the higher risk of lymphoma in this patient, a thoraco-abdominopelvic computed tomography (CT) scan revealed a triangular homogenous mass on anterior mediastinum, with 37x19 mm (Fig. 1), suggesting TH, hepatomegaly (16,1 cm on middle-clavicular line) and slight splenomegaly (bipolar diameter of 13,3 cm). Anti-acetylcholine receptor antibodies were negative, and thoracic magnetic resonance imaging (MRI) confirmed a triangular homogenous mass on anterior mediastinum, with 39x19 mm and preserved adjacent fat planes, compatible with TH (Fig. 2).
We opted for watchful waiting and close follow-up. The patient remained asymptomatic, abdominal ultrasound three months later showed a morphologically and dimensionally normal liver and spleen. Thoracic MRI six months later showed dimension stability of anterior mediastinal mass. Analytical reassessment showed persistent relative lymphocytosis without morphologically alterations. One year later CMV serology showed similar results.
DISCUSSION
Lymphocytosis is a common and inespecific laboratory finding related to multiple conditions. In patients with RA a variety of hematologic abnormalities may occur due to disease activity, pharmacological adverse effects, infection and many other causes. Common findings associated with active disease include anemia of chronic disease, thrombocytosis and sometimes a mild leukocytosis mandating exclusion of bacterial infection.7
In our patient’s epidemiological context, those symptoms along with relative lymphocytosis could be related to viral infection such as CMV mononucleosis. However, four months later, relative lymphocytosis remained. Being a young patient with RA, lymphoma should be excluded.2
The diagnostic work-up including CT scan showed an anterior mediastinal mass, suggesting TH. Anterior mediastinal masses include a variety of different entities demonstrating a spectrum of clinical and pathologic features. So, whether to pursue specific laboratory tests, look for specific associated conditions, obtain additional imaging or biopsy depends on which entities are realistic probabilities. Age and gender are the two most important initial features to consider in the evaluation of these patents.1
In asymptomatic men 10 to 39 years of age, thymoma and benign teratomas are the most common anterior mediastinal tumors. Other entities include lymphoma (Hodgkin´s disease, lymphoblastic non-Hodgkin´s lymphoma), seminoma or non-seminomatous germ cell tumor).1
Since certain entities manifested with anterior mediastinal masses present with highly characteristic clinical symptoms or findings, not all lesions in the anterior mediastinum need biopsy.1 On the basis of key imaging findings, TH can often be differentiated from neoplasm as the former usually presents with diffuse, symmetric thymic enlargement, a smooth contour, interspersed fat and soft-tissue elements, normal vessels, and preserved adjacent fat planes. Alternatively, a neoplasm may demonstrate a focal mass, a nodular contour, heterogeneity (ie, hemorrhage or necrosis), or calcifications.6,8 Thoracic MRI using chemical shift techniques is helpful in differentiating TH from thymomas and other anterior mediastinal tumors, with TH typically demonstrating a decrease in signal on opposed-phase images when compared with in-phase images.1, 5, 9 In this patient CT scan and MRI showed a homogeneous mass with preserved adjacent fat planes, morphologically compatible with TH.
TH being a benign lesion, is included in the group of entities responsible for approximately 5% of all thymic masses.1 There are two histologic types of TH: true TH and lymphoid follicular hyperplasia. The former is usually seen as a rebound phenomenon, characterized by an increase in mass of the gland after a stressor, such as chemotherapy, radiation, steroid treatment, burns, or surgery. Lymphoid follicular hyperplasia is associated with chronic inflammatory and autoimmune disorders, including myasthenia gravis (MG), Graves disease, systemic lupus erythematosus (SLE), RA, scleroderma, and other autoimmune conditions.5, 6, 8-10
Because of the highly frequent association of thymic abnormalities (such as thymoma and TH) and serum positivity to anti-acetylcholine receptor antibodies (approximately 40 to 75% of patients) these antibodies must always be examined in a patient diagnosed to have a thymic enlargement.11,12 In our patient, neurologic symptoms and anti-acetylcholine receptor antibodies were absent.
To our knowledge there are very few descriptions in the literature of cases of patients with TH in association with RA.13 However the identification of TH in patients with other autoimmune diseases suggests an autoimmune-mediated basis for TH with formation of medullary lymphoid follicles.9 This association was reinforced by Tellez-Zenteno JF et al 14 when evaluating the association of MG and connective tissue diseases (CTD). In a cohort of 132 patients with established diagnosis of MG undergoing thymectomy, 5% (6/132) of patients had CTD, of which five had RA and one SLE.
Given the benign nature of TH and the possible relationship with immunological mechanisms, it is reasonable to proceed with treating the autoimmune disease and follow-up the mass.1, 9 Thymic biopsy may be considered in cases of absence of regression when that is expected (when directed treatment to autoimmune disease is implemented) or growth. Surgical resection should be reserved for cases of significant associated MG.1
The association between mononucleosis and TH is not firmly stablished. However as long as the thymus plays an important role in cellular immunity in response to viral infections, we cannot exclude the contribution of an acute CMV infection to the development of TH.
CONCLUSION
This particular case described the association between TH and RA, in spite of clinical and serological remission. TH is a benign entity, requiring a conservative approach including clinical and radiological follow up. However, the differential diagnosis with conditions potentially more harmful, like lymphoma, is mandatory.
Figura I
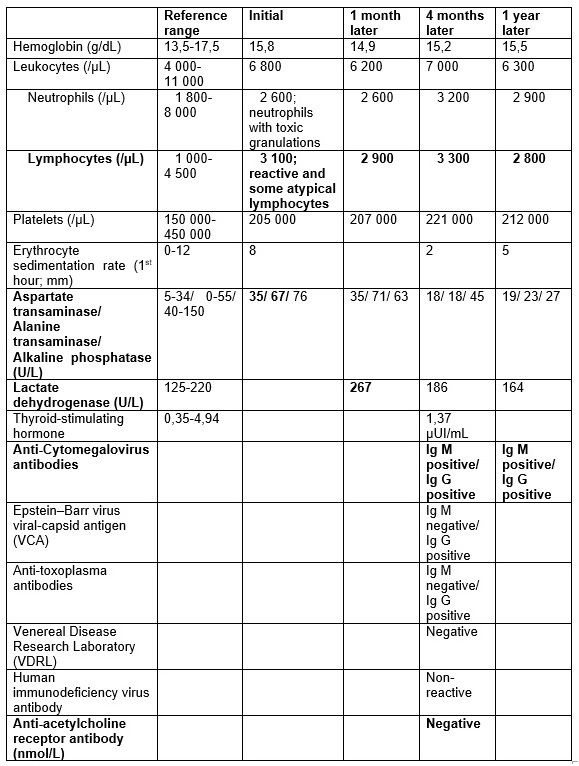
Table 1 – Laboratory tests
Figura II
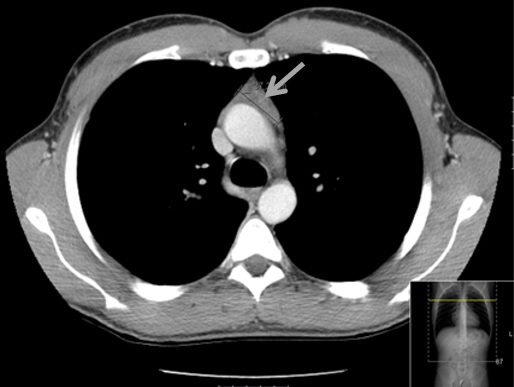
Figure 1 – Thoracic computed tomography: triangular homogenous mass on anterior mediastinum, with 37x19mm.
Figura III
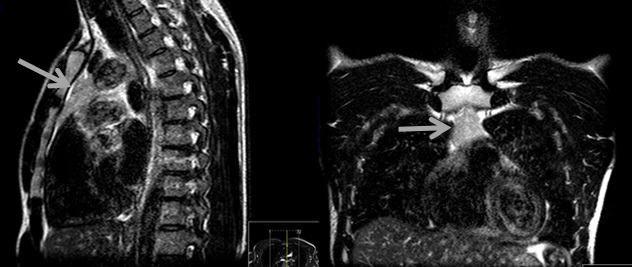
Figure 2 - Thoracic magnetic resonance imaging: triangular homogenous mass on anterior mediastinum, with 39x19 mm and preserved adjacent fat planes, compatible with thymic hyperplasia.
BIBLIOGRAFIA
1- Carter BW, Marom EM, Detterbeck FC. Aproaching the Patient with an Anterior Mediastinal Mass: A Guide for Clinicians. J Thorac Oncol. 2014; 9: S102–S109
2- Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015; 17:212
3- Askling J, Fored CM, Baecklund E, Brandt L, Backlin C, Ekbom A, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005; 64:1414–1420
4- Baecklund E, Smedby KE, Sutton LA, Askling J, Rosenquist R. Lymphoma development in patients with autoimmune and inflammatory disorders – What are the driving forces?. Semin Cancer Biol. 2014; 24: 61– 70
5- Goldstein AJ, Oliva I, Honarpisheh H, Rubinowitz A. A Tour of the Thymus: A Review of Thymic Lesions With Radiologic and Pathologic Correlation. Can Assoc Radiol J. 2015; 66: 5e15
6- Nishino M, Ashiku SK, Kocher ON, Thurer RL, Boiselle PM, Hatabu H. The Thymus a comprehensive review. Radiographics. 2006; 26(2):335-48
7- Grassi W, Angelis RD, Lamanna G, Cervini C. The clinical features of rheumatoid arthritis. Eur J Radiol. 1998; 27: S18–S24
8- Shields TW, LoCicero J, Reed CE, Feins RH. General Thoracic Surgery. 7th ed. Philadelphia: Lippincott Williams & Wilkins, 2011: 2059
9- Haider U, Richards P, Gianoukakis AG. Thymic Hyperplasia Associated with Graves´ Disease: Pathophysiology and Proposed Management Algorithm. Thyroid. 2017; 27(8):994-1000
10- Minato H, Kinoshita E, Nakada S, Nojima T, Tanaka M, Usuda K, et al. Thymic lymphoid hyperplasia with multilocular thymic cysts diagnosed before the Sjögren syndrome diagnosis. Diagn Pathol. 2015; 10:103
11- Fujii Y. The thymus, thymoma and myasthenia gravis. Surg Today. 2013; 43: 461–466
12- Chen P, Wang YP, Mou DL, Li ZY, Qu QM, Wang HY, et al. Pathological Findings in Myasthenia Gravis Patients with Thymic Hyperplasia and Thymoma. Pathol Oncol Res. 2018; 24(1):67-74
13- Sawada J, Asanome A, Endo H, Saito T, Katayama T, Hasebe N. A case of myasthenia gravis following sarcoidosis and rheumatoid arthritis. Rinsho Shinkeigaku. 2013; 53(5):351-5
14- Tellez-Zenteno JF, Remes-Troche JM, Mimenza-Alvarado A, Garcia-Ramos G, Estañol B, Vega-Boada F. The association of myasthenia gravis and connective tissue diseases. Effects of thymectomy in six cases with rheumatoid arthritis and one case with systemic lupus erythematosus. Neurologia. 2003;18 (2):54-8




