Introduction
Drug-induced liver injury (DILI) is defined as a liver injury that can be attributed to a medication, herb or xenobiotic, with resultant abnormalities in liver enzymes or liver dysfunction, after reasonable exclusion of other etiologies.1
DILI is the most common cause of acute liver failure in the USA and Europe, even though it represents less than 1% of cases of acute liver injury.2
The mechanisms of DILI are classified as intrinsic and idiosyncratic. Idiosyncratic DILI is almost impossible to predict. It affects only susceptible individuals, has less consistent relationship to dose, a more varied presentation and a variable latency to onset (weeks to months).3-5
Spironolactone is an aldosterone receptor antagonist and potassium-sparing diuretic widely used in the therapy of cirrhosis and cardiac failure. Spironolactone has been linked to rare cases of clinically apparent DILI, in patients taking doses of 50 and 100 mg/day. The authors report a case of liver injury induced by Spironolactone in a patient taking a dose of 25 mg/day.
Clinical Case
An 87-year-old man, with Parkinson’s disease, was referred to the Emergency Room with a history of sudden appearance of jaundice and a pruritic rash, affecting the back, chest, abdomen, arms and legs, with 24-48 hours of evolution. The patient also complained of nausea and vomiting. On admission, he was afebrile and stable, but presented with jaundice of scleras and skin, and with a diffuse maculopapular rash, affecting the described areas, that disappeared when pressed. Abdomen was innocent, as was the rest of his physical examination.
His laboratory findings showed a cholestatic pattern of liver injury (R value of 0.75): total bilirubin (TB) 302 µmol/L, with conjugated bilirubin of 217.9 µmol/L; alkaline phosphatase (ALP) 1148 U/L; gamma-glutamyl transferase (GGT) 1069 U/L; aspartate aminotransferase (AST) 852 U/L; alanine aminotransferase (ALT) 470 U/L. C-reactive protein, serum eosinophil percentage, amylase and lipase were normal. His abdominal ultrasound and CT were normal, making our main diagnostic hypothesis of biliary obstruction less likely. Despite this, the patient was admitted, and then submitted to a magnetic resonance cholangiopancreatography, also normal. Different laboratory studies were performed, to exclude other potential diagnoses: hepatitis A, B, C and E serologies; CMV, HSV, EBV and HIV serologies; serum proteinogram and immunoglobulin levels; autoantibodies (antinuclear; anti-smooth muscle; anti-liver kidney microsomal antibodies; anti-soluble liver antigen; anti-mitochondrial); copper serum level; and transferrin saturation.
During the 1st week of hospitalization, the patient’s liver enzymes showed a progressive fall, except for the TB, that reached a value of 546.5 µmol/L. Clinically, no more nausea or vomit occurred, but the patient’s jaundice (expectably) aggravated, the same happening to the rash, with appearance of pustules, that raised the possibility of an Acute Generalized Exanthematous Pustulosis (AGEP), confirmed with a skin biopsy.
Given the clinical picture and the absence of other apparent causes to it (all the laboratory study above mentioned was normal), DILI became our main diagnostic hypothesis. The patient was previously medicated with Levodopa/Carbidopa 200/50 mg 3 times/day for over a year. No other drugs (particularly antibiotics and pain killers), herbs or xenobiotics were consumed recently, except for Furosemide (60 mg/day) and Spironolactone (25 mg/day), both initiated 8 weeks before the beginning of the symptoms, for heart failure purposes. These drugs had been discontinued on the patient’s 1st day of admission and, apart from the TB increase, he was, indeed, showing some signs of improvement. However, on the 10th day of hospitalization, the transaminases suffered a marked rise, specially the AST (3019 U/L), a tendency maintained during the following days, with a switch in the pattern of liver injury (to a hepatocellular pattern; R value of 10.17). An ultrasound-guided liver biopsy was then performed, revealing no signs of chronic liver disease and a mixed pattern of liver injury with intrahepatic (hepato-canalicular) cholestasis, with spotty acidophilic necrosis (Fig. 1), suggesting two main differential diagnosis: toxic/drug induced or sepsis/systemic infection. Since the patient was never septic or infected, DILI was assumed as the cause of the liver disease.
Given the clinical history and temporality, both Furosemide and Spironolactone were considered as the main potential causes for this DILI. Based on LiverTox database, Spironolactone seemed the most probable drug involved, even more so after the restart of Furosemide without worsening of the clinical picture or the liver biochemistry. The diagnosis of DILI secondary to Spironolactone was then assumed. The patient evolved favorably and was discharge, after three months, with no jaundice, with normal levels of transaminases and TB, and with levels of ALP of 375 U/L (Fig. 2). The AGEP disappeared progressively without any specific treatment.
Discussion
The diagnosis of DILI can be difficult due to lack of specific signs, symptoms and tests, making it a diagnosis of exclusion. The clinical spectrum of DILI can mimic almost every other liver disorder, without symptoms that specifically point to the drug etiology unless rash or other cutaneous manifestations reinforce the suspicion of drug toxicity.6,7 This patient presented with jaundice, but with no other specific symptoms. The pruritic rash could have raised the possibility of DILI sooner, but there was no accompanying eosinophilia and still other hypothesis needed to be excluded.
Although not specific for DILI, ALT, AST, ALP and TB remain the hallmark for detecting liver damage and in the presence of this abnormal liver biochemistry, a diagnosis of DILI must always be considered, particularly if there is a history of recent onset of a new drug (usually in the previous 6 months).5 The diagnostic approach to DILI can be tailored according to the pattern of liver injury, given by the R-value (serum ALT/ALT upper limit of normal (ULN) divided by serum ALP/ALP ULN): ≥ 5 is labeled as hepatocellular DILI, < 2 as cholestatic, and 2 ≥ R < 5 as “mixed”.1,2,5 The pattern of injury can change over time but it provides a useful framework to allow one to focus on differential diagnosis and further evaluation.1 In this case, the initial R-value was 0.75, representing a cholestatic pattern, but within 10 days of admission, it evolved into a hepatocellular pattern (R-value of 10.17). After an extended analytical and imagiological study, DILI, even though it was always considered, turned into the main diagnostic hypothesis, particularly after the progression of the rash to an AGEP. This supported the diagnosis of DILI, since AGEP is an acute severe skin reaction characterized by the presence of an erythema and pustules that, similarly to DILI, is drug-related.8
A liver biopsy, usually not mandatory, was performed to corroborate the diagnosis and exclude other potential etiologies, since the patient aggravated his clinical and laboratory condition. Even though the patient showed a biochemical hepatocellular pattern of liver injury, the predominant pattern, in the biopsy, was hepato-canalicular intrahepatic cholestasis associated with spotty necrosis which demonstrates that the pattern of liver injury doesn’t always fully match the histologic picture.7
Despite the advances in recognizing DILI, its current diagnosis still depends on expert opinion, based on patient data and typical “signatures” associated with certain drugs. Accessing the cause of DILI can be even more challenging. Some complex scores (e.g., Roussel Uclaf Causality Assessment Method, RUCAM) were developed to improve DILI’s clinical and etiologic diagnosis.In this case, the RUCAM scores were calculated (Fig. 3) and even though they were similar between the three drugs possibly involved, Spironolactone showed a value of 8 (highly probable). Other facts support Spironolactone as the most probable cause of this DILI: Furosemide was restarted without worsening of the patient (negative re-challenge), which highly suggests that this was not the causative drug; Levodopa/Carbidopa, with a RUCAM score of 6, was being taken for over a year prior to the beginning of the symptoms, which makes this drug highly improbable as the causative agent, since the majority of DILI cases happens until 6 months after the beginning of a new drug. Also, according to LiverTox, clinically apparent liver injury induced by loop diuretics or Levodopa/Carbidopa is rare, if it occurs at all.9,10
In contrast, even though rare, there has been 3 reports of Spironolactone induced liver injury: 2 occurring with doses of 100 mg/day11,12 and one with 50 mg/day13 (being the clinical picture and progression of this particular case, extremely similar to the one the authors present). All the patients improved after discontinuation of the drug.
The mechanism of Spironolactone hepatic injury is unknown but is most likely due to a metabolic idiosyncrasy. Spironolactone inhibits cytochrome P450, possibly inducing microsomal enzymes that can lead to the formation of toxic metabolites and ultimately to hepatic injury, that typically arises after 4 to 8 weeks of therapy, being the pattern of serum enzyme elevations usually hepatocellular or mixed.14
In view of the above, the authors believe that a causal relationship between the described manifestations and Spironolactone is proven, and this case intends to alert to this rare but possible and serious toxicity, even when lower doses of the drug are used (this patient was under 25 mg/day for 8 weeks).
Finally, even though the main therapeutic measure in DILI (and AGEP) situations is the immediate withdrawal of the causative agent, the liver injury may still progress initially, as happened in this case. It’s important that the clinicians don’t assume an immediate diagnostic/therapeutic failure but rather monitor the patient’s clinical and laboratory condition closely and make every effort to exclude other potential etiologies.
Figura I
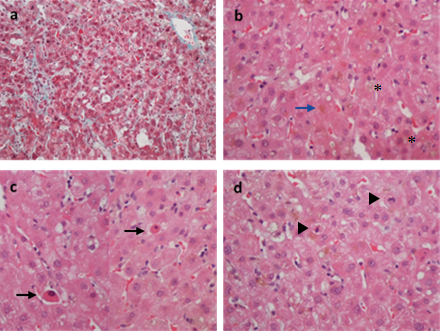
Figure 1. a Preserved liver architecture with normal portal tracts and no evidence of fibrosis (Masson Trichrome x 20) b Diffuse bilirubinostasis with bile plugs (asterisks) in canaliculi and bilirubin pigment in hepatocytes (blue arrow) (H & E x 40). c Numerous foci of spotty lobular necrosis with apoptotic bodies (arrows) (H & E x 40). d Mitotic activity (arrowheads) in response to hepatocellular damage (H & E x 40).
Figura II
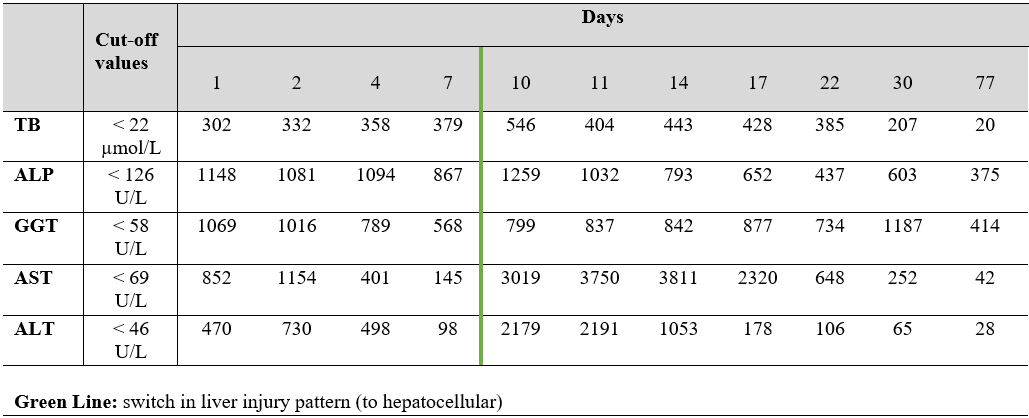
Figure 2. Temporal variations of relevant analytical parameters.
Figura III
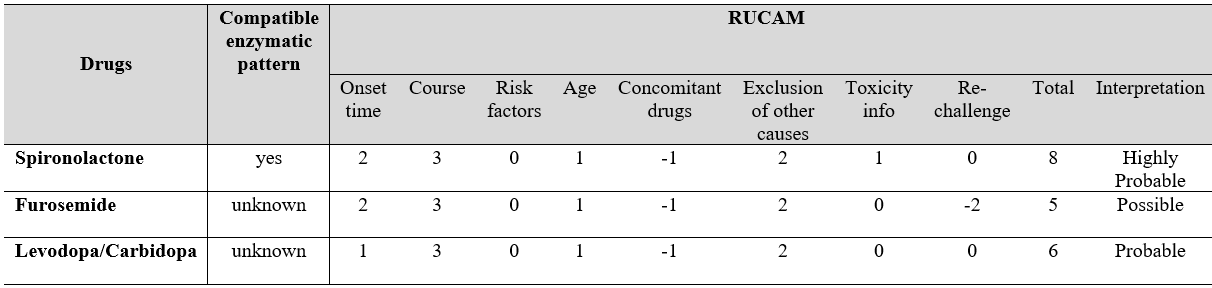
Figure 3. RUCAM assessment for the drugs the patient was under at the time of admission.
BIBLIOGRAFIA
References
1. Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clinical and Molecular Hepatology. 2012; 18:249-257.
2. Kullak-Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, et al. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut. 2017; 66:1154–1164.
3. Larson AM. Drugs and the liver: Patterns of drug-induced liver injury. UpTodate 2018. (accessed December 2018). Availiable from https://www.uptodate.com/contents/drugs-and-the-liver-patterns-of-drug-induced-liver-injury.
4. Larson AM. Drugs and the liver: Metabolism and mechanisms of injury. UpTodate 2018. (accessed December 2018). Availiable from https://www.uptodate.com/contents/drugs-and-the-liver-metabolism-and-mechanisms-of-injury.
5. Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG Clinical Guideline: The Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am J Gastroenterol. 2014; 109(7):950-66.
6. Padda MS, Sanchez M, Akhtar AJ, Boyer JL. Drug Induced Cholestasis. Hepatology. 2011; 53(4):1377-87.
7. Haque T, Sasatomi E, Hayashi P. Drug-Induced Liver Injury: Pattern Recognition and Future Directions. Gut Liver. 2016; 10:27-36.
8. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute Generalized Exanthematous Pustulosis:
Pathogenesis, Genetic Background, Clinical Variants and Therapy. Int. J. Mol. Sci. 2016; 17:1214.
9. National Institutes of Health, U.S. Department of Health & Human Services. LiverTox: Loop Diuretics [webpage]. Bethesda: U.S. National Library of Medicine; 2018 [accessed in August 2018]. Available in: https ://livertox.nlm.nih.gov/Diuretics.htm.
10. National Institutes of Health, U.S. Department of Health & Human Services. LiverTox: Levodopa and Carbidopa [webpage]. Bethesda: U.S. National Library of Medicine; 2018 [accessed in August 2018]. Available in: https://livertox.nih.gov/Levodopa_and_Carbidopa.htm.
11. Thai KE, Sinclair RD. Spironolactone-induced hepatitis. Australasian Journal of Dermatology. 2001; 42:180–182.
12. Shuk J, Shen S, Owensby L, Leftik M, Cucinell SS. Spironolactone hepatitis in primary hyperaldosteronism. Ann. Int. Med. 1981; 95:708–10.
13. Renkes P, Gaucher P, Tréchot P. Spironolactone and Hepatic Toxicity. JAMA. 1995; 273(5):376–377.
14. National Institutes of Health, U.S. Department of Health & Human Services. LiverTox: Spironolactone [webpage]. Bethesda: U.S. National Library of Medicine; 2018 [accessed in August 2018]. Available in: https://livertox.nih.gov/Spironolactone.htm.




