INTRODUCTION
Type 1 diabetes mellitus, a common organ-specific autoimmune disease that leads to a chronic deficiency of insulin, can be associated with other autoimmune diseases, more frequently thyroiditis and celiac disease. Dermatomyositis is a rare systemic autoimmune disease of unknown aetiology, characterized by inflammation of the estriated musculature and typical cutaneous manifestations, linked to a non-suppurative inflammatory process dominated by lymphocytic infiltration. Although autoimmune diseases are usually associated with each other, the presence of these two is rare, with very few cases reported in the literature1.
CASE PRESENTATION:
A 53-year-woman with type 1 diabetes mellitus diagnosed at age 27 with micro and macroangiopathic end-organ damage (retinopathy, chronic kidney disease stage 3, polyneuropathy (diabetic foot and bilateral carpal tunnel syndrome), glycemic instability HbA1c > 9.0% with hypoglicemia, arterial hypertension, dyslipidemia, normocytic normochromic multifactorial anemia, mild pulmonary hypertension, esophagitis and gastric vascular ectasia, mixed chronic pain and depressive disorder. Regular medications: NPHinsulin 16U + 18U and insulin aspartat meal times, candesartan 32mg id, furosemide 40mg id, carvedilol25mg id, simvastatin 40mg id, lercanidipine 20mg bid, tapentadol 50mg bid.
She noted depigmentation of the skin, itching, muscle pain, weight loss, dysphagia, dysphonia and decreased pelvic and shoulder girdles muscle strength, followed a year later by facial and member swelling (figure 1). She was admitted to the hospital fifteen months after the beggining of symptons. Relevant physical examination showed decreased proximal and distal symmetrical muscle strength grade 4/5; pain upon palpation of the upper limbs and lower limbs.
The overall study revealed: VS 17 mm, LDH 1555 UI/L, ALT/AST 128/162 UI/L, Total CK 3132 UI/L, myoglobin 7985 ng/mL, creatinine/urea 4,2/145 mg/dL. Positive antinuclear antibodies 1/2560 with dense fine granular pattern with mitosis. Positive anti-Mi-2 antibodies. Viral serologies and interferon gamma release assay were negative. Paraneoplastic study had no major findings.
Esophageal manometry showed inefficient esophageal motility. Electromyography showed fibrillations and positive waves.
Nail fold capillaroscopy showed a scleroderma pattern, which can also occur in dermatomyositis2. Muscle biopsy was compatible with dermatomyositis (figure 2).
Treatment with Prednisolone 1mg/Kg/day (75mg per os), Hydroxychloroquine 200mg/day and Methotrexate 10mg/week was instituted.
When methotrexate was started the patient developed severe pancytopenia and esophageal candidiasis and in order to replace it, endovenous immunoglobulin was used (2g/Kg, for 5 days). Azathioprine was then tried with success after genetic tests for TPMT (enzyme thiopurine methyltransferase).
DISCUSSION
Early diagnosis of Dermatomyositis is imperative because of the implications on prognosis from late start of treatment3,4. Initial therapy encompasses the use of high dose corticosteroids and for that this case proved to have an even greater challenge: reaching metabolic control while controlling inflammatory activity of Dermatomyositis. Furthermore, the important association of dermatomyositis with malignancy poses another challenge and difficulty in the use of steroids, since malignancy underlies dermatomyositis in about 8.5% of patients over the age of 40 and may precede or follow the onset of the myositis by up to 2 years5.
Particular causes of death related to dermatomyositis include muscle weakness, cardiopulmonary involvement or associated malignancy3,4. In this patient dysphagia confers a worse prognosis.
T1DM is not genetically predetermined, but an increased susceptibility to the disease may be inherited and the major histocompatability complex (MHC) class II alleles and human leukocyte antigen (HLA)-DR3 and DR4 found to be carried in 90% of the patients with T1DM5. Concomitant coexistence of diabetes mellitus and dermatomyositis is describedpoorly in the literature. A report revealed the presence of HLA antigens A2, A9, B8, B27, DR3 and DR4 as predicting factors5, but thereĺs little investigation to support it. Recent advances in immunology have identified another population of CD4+ T cells, Th17 cells, that have been demonstrated be pathogenic mediators of several autoimmune diseases once attributed to Th1 cells such as T1DM6. The pathophysiology of T1DM and DM involves both Th1 and Th17 inflammatory responses, they both occur with lymphocytic infiltration of the organs affected, however they rarely overlap.
Unlike other autoimmune diseases the association between T1DM with dermatomyositis is extremely rare, so far, for unexplained reasons. It may be that type 1 diabetics are less likely to have dermatomyositis however, since T1DM is a common autoimmune disease and dermatomyositis like other autoimmune myositis are rare entities, there is no data to support this hypothesis yet. Environmental factors that predispose to both manifestations may justify the infrequency of overlapping but currently the cohorts of immune myositis are poorly studied and still less structured, with respect to comorbidities and the respective publication of these registries.
Varying degrees of overlapping autoimmune diseases exist and the clinician should also be aware of dermatomyositis to make an early diagnosis.
Figura I
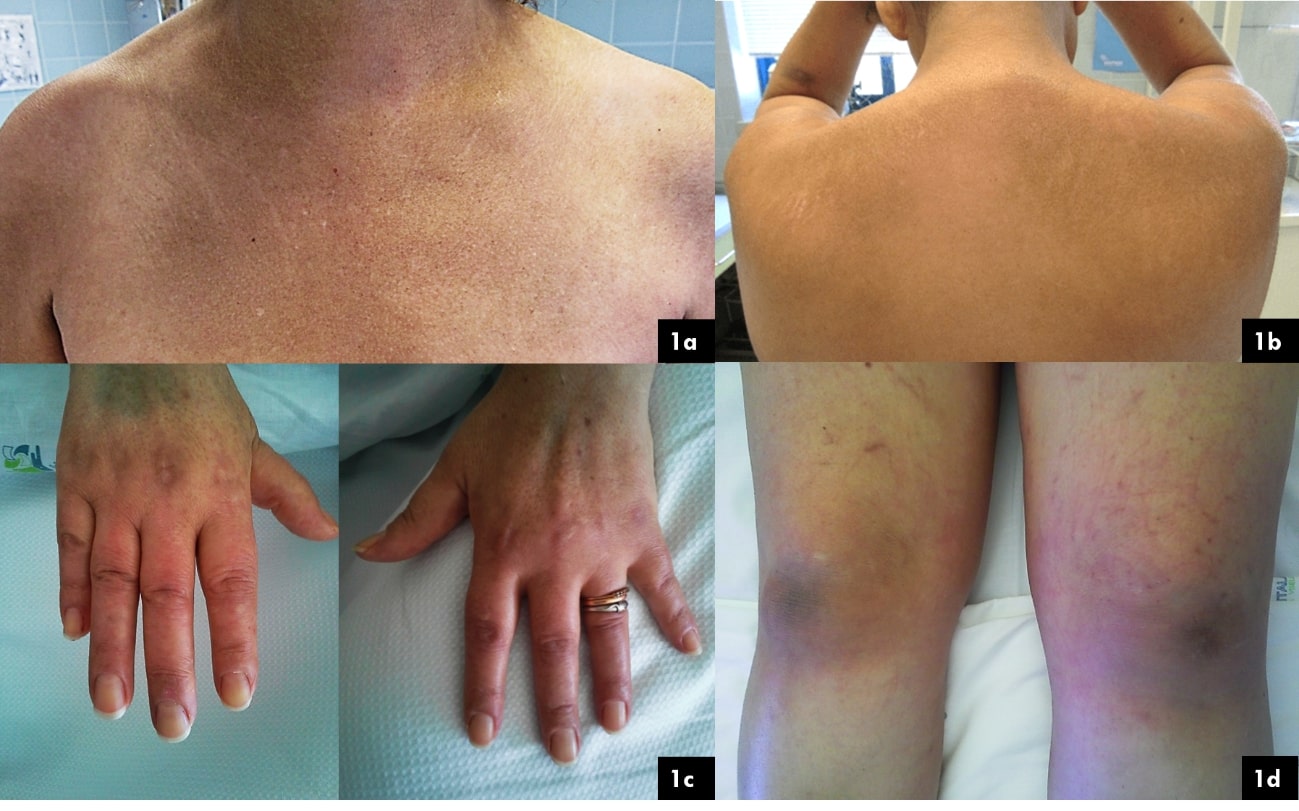
Figure 1: 1a ľ ôV neckö sign: erythematous and hyperpigmented macules on the chest; 1b ľ Shawl sign: erythema over the upper back, posterior neck, and shoulders (classic in dermatomyositis); 1c ľ Gottronĺs papules: violaceous, scaling papules on the skin overlying the joints of the hand and proximal nailfolds (a pathognomonic sign of dermatomyositis); 1d ľ Gottronĺs sign: violaceous patches overlying the knees.
Figura II
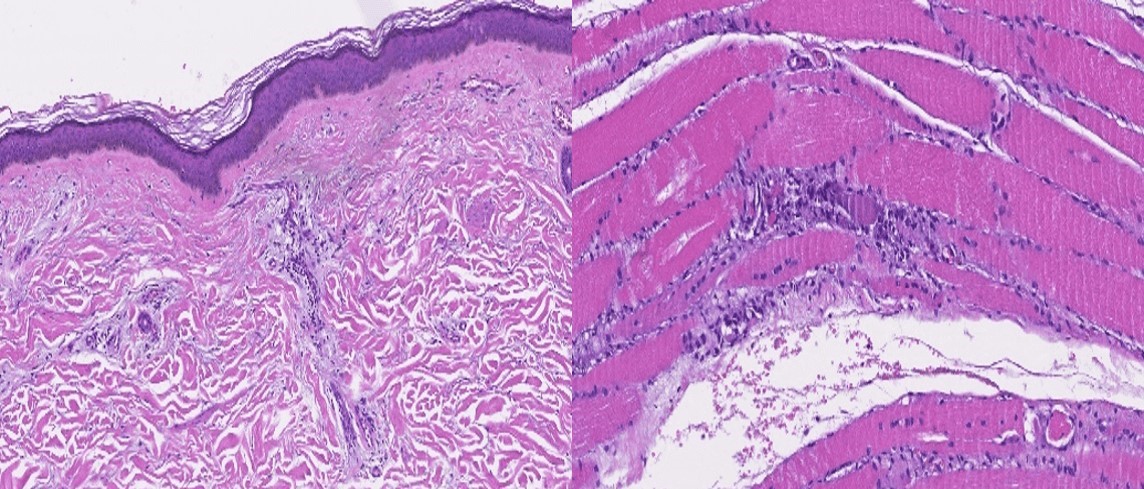
Figure 2: Muscle biopsy (hematoxylin and eosin stain) showing skin atrophy, perivascular inflammation, capillary damage and atrophy in perifascicular myofibers.
BIBLIOGRAFIA
1. Shanker K, Daley T, Semple R, Rouster-Stevens K, Ham JN. Intractable Hypoglycemia in the Setting of Autoimmune Overlap Syndrome. Pediatrics [Internet]. 2017;139(6):e20160866.
2. Rosßrio e Souza EJ do, Kayser C. Capilaroscopia periungueal: RelevÔncia para a prßtica reumatolˇgica. Rev Bras Reumatol [Internet]. 2015;55(3):264ľ71.
3. Yamasaki M, Yamada H. Polymyositis and dermatomyositis. Nippon Rinsho [Internet]. 1999;57(2):339ľ43.
4. Jury EC, DĺCruz D, Morrow WJW. Autoantibodies and overlap syndromes in autoimmune rheumatic disease. J Clin Pathol. 2001;54(5):340ľ7.
5. Charalabopoulos K, Charalabopoulos A, Papaioannides D. Diabetes mellitus type I associated with dermatomyositis: an extraordinary rare case with a brief literature review. BMJ Case Rep. 2009;bcr10.2008.
6. Solt L, Burris T. Th17 cells in type 1 diabetes: a future perspective. Diabetes Manag [Internet]. 2015;5(4):247ľ50.



