INTRODUCTION
Renal papillary necrosis (RPN) is a condition that may arise from various diseases that induce chronic tubulointerstitial nephropathy predominantly affecting the inner medulla. It is characterized by impairment of the vascular supply and from subsequent focal or diffuse ischemic necrosis of the distal segments of the renal pyramids, which are particularly vulnerable to ischemic insults because of their blood supply and the hypertonic environment surrounding them. The vasa recta form wide vascular bundles at the base of the medullary pyramid, but the bundles taper as they continue distally toward the apex and papilla, resulting in a marginal blood supply to the papillary tip - a predisposing factor for ischemia and the subsequent development of renal papillary necrosis 1.
Various conditions have a known association with renal papillary necrosis: analgesic nephropathy is considered the most common causative factor, and results from the daily use, heavy or not, for many years of preparations containing at least two analgesics (acetylsalicylic acid, acetaminophen, phenacetin, pyrazolones) and central-acting dependence-inducing substances (caffeine, codeine, and/or barbiturates) 2. Other causes include Diabetes mellitus, sickle cell disease, tuberculosis and renal vein thrombosis. On the other hand, any condition associated with ischemia predisposes an individual to papillary necrosis such as shock, hypovolemia and hypoxia 1,3.
CASE DESCRIPTION
The authors describe the case of a 38-year-old woman with history of major depressive disorder, multiple previous suicide attempts and an addictive behaviour with chronic abuse of benzodiazepines, acetaminophen and codeine with progressive dosage increase over the last ten years. She had an estimated daily consumption of 20mg of alprazolam, 5g of acetaminophen and 300mg of codeine.
She was admitted in an intensive care unit (ICU) after voluntary drug overdose with 15g of acetaminophen, 900mg of codeine, 3g of venlafaxine, 40mg of alprazolam and 9g of quetiapine that led to coma. On admission to the ICU she had multiorgan failure, with severe neurologic, respiratory, cardiovascular and hepatic dysfunctions and supportive care was provided. Vasopressorsupport with norepinephrine was required during the first five days of hospital stay, due to the severe distributive shock the patient had. ICU stay was complicated by nosocomial pneumonia with severe respiratory failure, with subsequent initiation of broad spectrum antibiotics on day six of hospital stay. Antibiotics were tapered to amoxicillin/clavulanate as soon as multi-sensitive Staphylococcus aureus was isolated in blood cultures, and were continued for a total of two weeks. Sedation with fentanyl and midazolam were used initially, the latter replaced for propofol after resolution of distributive shock, and was administered for additional eight days. Delirium and agitation as well as unilateral paralysis of vocal cords hampered weaning from mechanical ventilation. Non-invasive ventilation, quetiapine (up to 800mg per day) and alprazolam (up to 16mg per day) were employed with successful extubation on the fifteenth day of hospital stay. Acute liver injury with a cholestatic pattern was probably multifactorial in its genesis and improved throughout the inicial days of hospitalization. Renal function and urine output were normal throughout intensive care stay and no imaging study was carried out. Clinical stability enabled the transfer to an internal medicine ward on day seventeenth day of hospital stay.
On admission to the internal medicine ward she was alert, with a depressed mood, nodding “yes” or “no” to all questions. Her vital signs were stable, and her blood work was unremarkable apart from minor cholestasis (in a decreasing trend) and a stable normochromic normocytic anaemia with normal creatinine and urea levels.
On the 21st day of hospitalization, the on-call physician was called due to patient’s agitation. Physical examination was unremarkable. Anuria had evolved over the last 16 hours and a urinary catheter was put in place with 200cc of fresh red blood coming out. Emergency blood work showed acute kidney injury with creatinine level of 5.76 mg/dL (normal range 0.51-0.95) and urea of 125 mg/dL (normal range 10-50). Reno-pelvic ultrasound showed mild bilateral uretero-hydronephrosis. Computed tomography revealed mild bilateral hydronephrosis with hyperdense foci in the pelvicaliceal system suggesting haemorrhagic content. After intravenous contrast administration, excretory phase CT depicted multiple filling defects in the left renal collecting system which in this clinical context were suggestive of sloughed papillae due to papillary necrosis (Fig. 1).
The patient underwent a cystoscopy, with hematic urine and small clots being visualized in the bladder. Retrograde pyelography showed bilateral pelvicalyceal and ureteral dilatation, as well as filling defects in the pelvicalyceal system. Continued postoperative ureteric drainage was ensured by the insertion of bilateral double-J stents. Postoperatively, the patient became polyuric during the first 48 hours but normalization of urine output and renal functions occurred after 4 days. Urinary and blood cultures were negative. Posterior follow-up revealed normal renal function, and elective removal of the double-J stents was performed 4 weeks later.
DISCUSSION
In renal papillary necrosis, renal papillae may undergo complete or partial necrosis and the necrotic papillae may remain within the cavity, be absorbed, passed in the urine, or calcify. Sloughed papillae may cause acute ureteral obstruction resulting in acute kidney injury with gross haematuria, oliguria or anuria, although this is a rare presentation. More frequently there is an insidious course over months to years, with little or no symptoms 1,3.
Radiological diagnosis of RPN relies on demonstrating papillary/calyceal abnormalities and intravenous urography is the method of choice, with other imaging studies like ultrasound and CT scan being less sensitive. Retrograde pyelography, albeit more invasive, is useful when the renal collecting system opacifies poorly with intravenous contrast or when there is kidney injury 3.
This case illustrates a case of acute RPN with a rare presentation with bilateral ureteric obstruction due to the sloughed papillae, warranting urgent ureteric drainage. The most likely cause of the RPN in this case was an overlap phenomenon where multiple renal insults occurred simultaneously in a patient with a history of heavy analgesic abuse and possibly analgesic nephropathy: acute ingestion of a large amount of acetaminophen and codeine, vasoplegic shock with the need of vasopressor support as well as the possible contribution of hypoxemia from the nosocomial pneumonia with concomitant bacteraemia. Another possible contributor might be the possible existence of interstitial nephritis related to the antibiotherapy performed with amoxicillin/clavulanate (although there was no evidence of eosinophilia or eosinophiluria, and no renal biopsy was performed to confirm this diagnosis).Although there have been cases in the literature in which papillary necrosis occurred more than one week after the primary insult 4,5, the reason why RPN occurred after the stabilization of the patient remains unclear.
The prognosis of RPN depends on the aetiology of the ischemic insult, the degree of the necrosis, the involvement of one or both kidneys, and the overall health status of the patient 1. In this case, normalization of urine output and function occurred, nevertheless given the history of heavy analgesic abuse, it is paramount their use be evicted, whilst also avoiding dehydration and monitoring renal function periodically.
Figura I
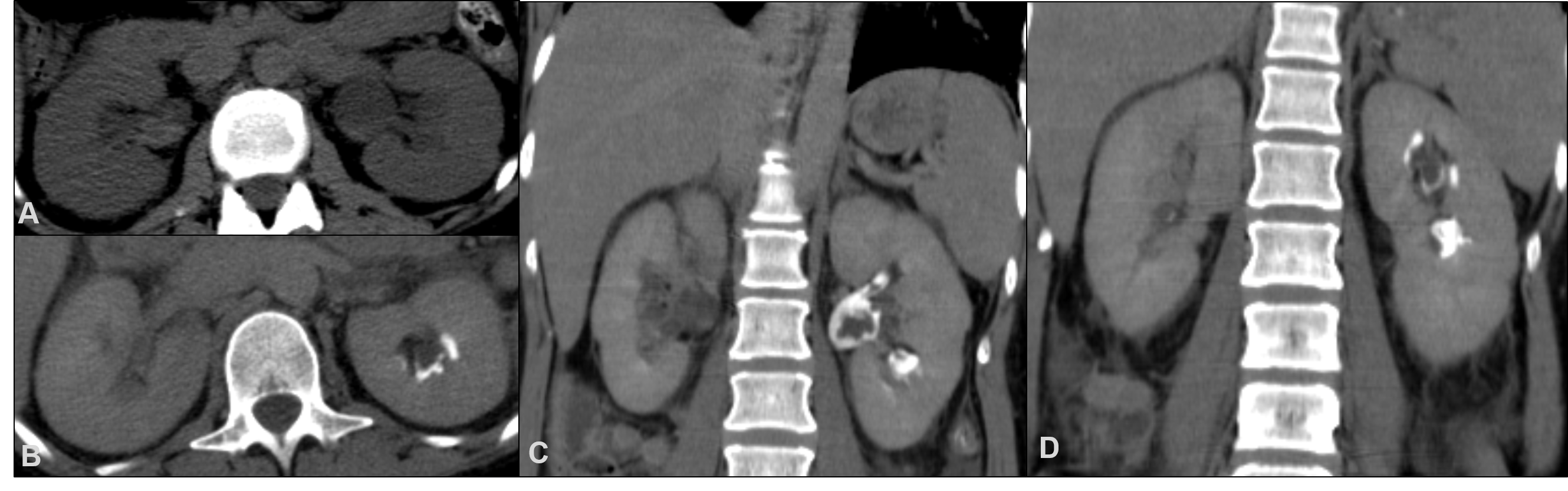
Figure 1. Plain CT (A) show mild bilateral hydronephrosis and hyperdense foci in both pelvicaliceal systems, suggesting haemorrhagic content. CT-urography in axial (B) and coronal planes (C, D) reveal multiple filling defects in the left renal collecting system suggestive of sloughed papillay due to papillary necrosis.
BIBLIOGRAFIA
1. Jung DC, Kim SH, Jung SI, Hwang SI, Kim SH. Renal papillary necrosis: review and comparison of finding at multi-detector row CT and intravenous urography. Radiographics 2006; 26:1827-1836.
2. De Broe ME, Elseviers MM. Over-the-counter analgesic use. J Am Soc Nephrol. 2009; 20:2098-103
3. Sutariya HC, Pandya VK. Renal Papillary Necrosis: Role of Radiology. J Clin Diagn Res. 2016; 10: TD10-2
4. Kovacevic L, Bernstein J, Valentini RP, Imam A, Gupta N, Mattoo TK. Renal papillary necrosis induced by naproxen. Pediatr Nephrol. 2003; 18:826-9
5. Witting MD. Renal papillary necrosis following emergency department treatment of migraine. J Emerg Med. 1996; 14:373-6


