INTRODUCTIONEssential thrombocythemia (ET) is a rare, chronic myeloproliferative neoplasm characterized by sustained elevation of platelet count due to megakaryocyte bone marrow expansion in the absence of a definable cause
1. ET is usually diagnosed in middle-aged males and females in equal proportions
2 and is associated with an increased risk of thrombosis, hemorrhage and vasomotor symptoms. Thrombosis can occur at any location in the venous or arterial beds, including the coronary arteries
2,3. The incidence of acute coronary syndromes (ACS) in ET patients was reported under 10% and is more frequent over age 40
4. Here, we present two patients with ET admitted with an ACS in a tertiary hospital for the past 5 years.
CASE REPORTSPATIENT AA 32-year-old male was admitted with sudden-onset chest pain, which started 5 hours before. He had ET for 9 years but was lost to follow-up and treatment an year after diagnosis. He quit smoking one year before (17 pack-years) but had no other major cardiovascular (CV) risk factors (Table 1). Physical examination was unremarkable but as the ECG showed ST segment elevation in the anterior leads (V1-V6), he was diagnosed with an anterior ST-segment elevation myocardial infarction (STEMI) in the context of ET. A loading dose of ASA (300 mg) and ticagrelor (180 mg) was administered. The patient underwent emergent coronary angiography which showed a 50% left main (LM) stenosis and a critical, proximal left anterior descending (LAD) stenosis with TIMI 3 flow (Fig.1). After Heart Team discussion, the patient was referred to coronary artery bypass graft (CABG) surgery. Platelet counts were persistently elevated (admission: 676×10
9/L; discharge: 632×10
9/L). Hemoglobin (14,3 g/dL), hematocrit (41%) and coagulation studies (INR 0,9; aPTT 26) were normal. Echocardiography showed preserved left ventricular ejection fraction (LVEF 58%) but with anterior wall hypokinesis. At discharge, he was treated with dual antiplatelet therapy (DAPT) including ASA (100 mg od) and ticagrelor (90 mg bid), hydroxyurea and was referred to follow-up in Hematology and Cardiology. After 6 months, no other thrombotic complications were observed, and the platelets decreased to 583x10
9/L (Table 1).
PATIENT BA 47-year-old male was admitted with persistent chest pain for 12 hours. He had been diagnosed with ET 5 years before (Table 1) and was treated with low dose (100mg) ASA. No major CV risk factors were found (Table 1). Physical examination was normal but the ECG showed ST segment elevation in the anterior leads (V2-V5), and the patient was diagnosed with an anterior STEMI in the context of ET and a loading dose of ASA and ticagrelor was administered. The coronary angiography showed a critical LAD stenosis in the followed by a distal occlusion (Fig.2). He was successfully submitted to thrombus aspiration followed by a balloon angioplasty and DAPT including ASA and ticagrelor was instituted. Platelet counts were persistently elevated (admission: 945×10
9/L; discharge: 920×10
9/L). Hemoglobin (13,0 g/dL), hematocrit (42%) and coagulation studies (INR 1,0; aPTT 32) were normal. The echocardiography showed a preserved LVEF (>55%) but with anterior wall hypokinesis. At discharge, he was treated with DAPT (ASA + ticagrelor), hydroxyurea and was referred to follow-up in Hematology and Cardiology consults. After 6 months, no other thrombotic complications were observed, and the platelet count decreased to 786x10
9/L (Table 1).
DISCUSSIONThe revised diagnostic criteria for ET include elevated platelet count (>450x10
9/L) and bone marrow megakaryocytes proliferation, with (i) either a genetic mutation (JAK2, CALR or MPL), (ii) the presence of a clonal marker or (iii) absence of evidence for reactive thrombocytosis
1. JAK2, CALR, and MPL mutations are the mutually exclusive mutations in ET with respective incidences of 55%, 25%, and 3%, while approximately 17% are triple‐negative. In ET, mutant CALR (vs JAK2) was associated with younger age, male sex, higher platelet count and lower incidence of thrombotic events
3. Both patients met the diagnostic criteria and fit the demographic pattern.
The management of ET aims to prevent thrombotic complications, without increasing bleeding risk, and secondarily to control microcirculatory symptoms. In this regard, treatment is tailored according to the presumed risk for thrombosis or bleeding. Thrombotic risk stratification includes four categories: very low risk (age ≤60 years, no thrombosis history, JAK2/MPL un‐mutated), low risk (age ≤60 years, no thrombosis history, JAK2/MPL mutated), intermediate risk (age >60 years, no thrombosis history, JAK2/MPL un‐mutated) and high risk (thrombosis history or age >60 years with JAK2/MPL mutation)
3. In contrast, hemorrhagic risk increases proportionally with platelet counts of above 1000x10
9/L due to acquired von-Willebrand syndrome (AvWS)
3. Even at normal platelet counts a clinically relevant AvWS can occur
3. Thus, a laboratory evaluation including ristocetin cofactor activity for AvWS is recommended in the presence of abnormal bleeding or extreme thrombocytosis
3.
Treatment is based on effective platelet inhibition and cytoreduction. Low‐dose ASA (range 40‐100mg/day) is recommended in most ET patients, provided there are no major contraindications (ristocetin cofactor activity <20%‐30%)
3. In ASA‐resistant symptoms, it is reasonable to utilize a twice‐daily regimen of low dose ASA or alternative anti‐platelet agents such as clopidogrel (75 mg/day) alone or in combination with ASA
3. Moreover, it is recommended the use of platelet‐lowering agents in ASA‐refractory cases even with low risk, but the target platelet count in this setting should be the relief of symptoms, and not necessarily 400 × 10
9/L
3. Prior to the thrombotic event, at least one patient was undertreated.
In a large study of ET patients, 9% presented with arterial thrombosis, within a median follow-up of 6.2 years
4. STEMI is an uncommon complication of ET, which is associated with older age (> 60 years), history of thrombotic events, leukocytosis, presence of cardiovascular risk factors and JAK2 mutation
3, which our patients did not present. Optimal treatment modalities of ET patients with STEMI mostly derive from case reports but ET treatment is key.Cytoreductive treatment with hydroxyurea (starting dose 500mg BID) should be initiated preferably before coronary intervention to prevent no-reflow caused by platelet aggregation and activation
5. Alternatively, patients resistant or intolerant to hydroxyurea are managed with pegylated interferon (IFN)‐α (45 mcg once‐a‐week and titrate up to 180 mcg) or busulfan (4 mg/day). IFN therapy is preferred in younger patients and was associated with significant reduction in mutant CALR allele burden. Anagrelide was associated with higher risk of arterial thrombosis
3. Despite not being evidence based, the target in high risk ET patients should be a normal platelet count
3. Neither patient met the platelet count target mostly due to inconsistent therapeutic compliance.
In an ET-related STEMI, standard antithrombotic therapy is chosen according with ongoing antiplatelet treatment and bleeding risk. The main difference resides in the maintenance period after one year of DAPT. Patients who are over age 60, JAK2-mutated, have cardiovascular risk factors or previous treatment with ASA, are candidates to a twice-daily ASA regimen. Alternative regimens including clopidogrel or ticagrelor in monotherapy, or in combination with ASA may be considered on the long term, as data is emerging on the efficacy and safety of prolonged DAPT therapy
3.
Coronary reperfusion should be performed as early as possible. Our patients were successfully treated with different approaches (PCI and CABG). However, there is some debate on the adequate vessel lesion approach due to the different pathophysiological mechanisms involved.
Quadro I
Summary of patient characteristics
| | | |
| | Patient A | Patient B |
| Age (years) | 32 | 47 |
| Sex | Male | Male |
| Arterial Hypertension | No | No |
| Diabetes | No | No |
| Fasting glycemia (mg/dL) | 94 | 75 |
| Hemoglobin A1C (%) | 5,8 | 5,2 |
| Dyslipidemia | No | No |
| Total cholesterol (mg/dL) | 175 | 135 |
| Low-density lipoprotein (mg/dL) | 97 | 83 |
| High-density lipoprotein (mg/dL) | 62 | 44 |
| Triglycerides (mg/dL) | 93 | 63 |
| Cigarrete smoking | Former smoker (17 pack-years) | No |
| Family history of coronary artery disease | No | No |
| EKG ST segment elevation leads | V1-V6 | V2-V5 |
| High sensitivity Troponin I at admission (ng/L) | 7890 | 2340 |
| High sensitivity Troponin I peak (ng/L) | 9269 | 2500 |
| Left ventricular ejection fraction (%) | 58% | >55% |
| Wall motion reduction | Anterior wall, apex | Anterior wall, apex |
| Culprit vessels | left coronary artery (LCA), left anterior descending artery (LAD) | left anterior descending artery (LAD) |
| Lesion description | LCA (segment 5) 50% stenosis, <10mm; LAD (segment 6) 91-99% stenosis, 10-20mm | LAD (segment 6) 50% stenosis, 10-20mm; LAD (segment 8) occlusion, 10-20mm |
| Coronary reperfusion therapy | Coronary artery bypass surgery | Percutaneous coronary intervention |
| Time from ET diagnosis to STEMI (years) | 9 | 5 |
| ET treatment prior to STEMI | None | Low dose ASA |
| Platelet count at admission (×109/L) | 676 | 945 |
| Platelet count after 6 months (×109/L) | 583 | 786 |
| Bone marrow biopsy | Megakaryocyte hyperplasia with hyperlobated nuclei | Megakaryocyte hyperplasia with hyperlobated nuclei |
| Mutated gene | CALR | CALR |
| Mutation description | p.L367fs*46, deletion of 52 nucleotides (c.1099_1150del) on exon 9 of CALR gene | p.K368fs*46, deletion of 60 nucleotides, with insertion of 11 nucleotides (c.1101_1160delinsCCTTTGTCCTT) on exon 9 of CALR gene |
Figura I
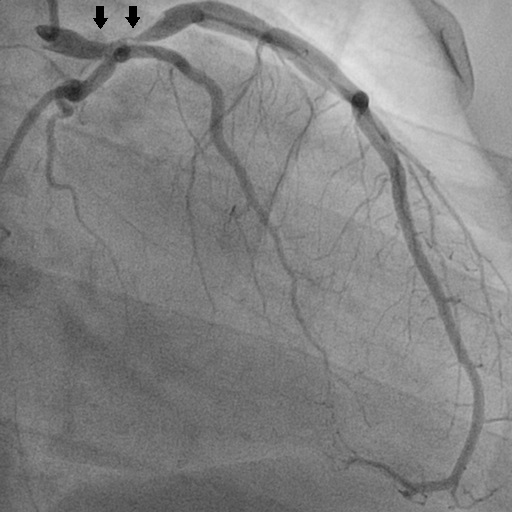
Coronary angiography of Patient A: left coronary artery (segment 5) 50% stenosis, <10mm; anterior descending artery (segment 6) 91-99% stenosis, 10-20mm.
Figura II
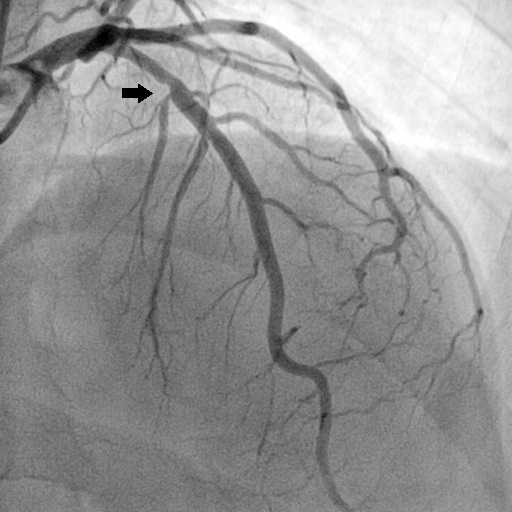
Coronary angiography of Patient B: anterior descending artery (segment 6) 50% stenosis, 10-20mm; anterior descending artery (segment 8) occlusion, 10-20mm.
BIBLIOGRAFIA
1. Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018 Feb 9;8(2):15.
2. Sanchez S, Ewton A. Essential thrombocythemia: A review of diagnostic and pathologic features. Vol. 130, Archives of Pathology and Laboratory Medicine. 2006. p. 1144–50.
3. Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. Am J Hematol. 2019 Jan;94(1):133-143.
4. Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, Rodeghiero F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011 Jun 2;117(22):5857 LP – 5859.
5. Fujimura M, Akaike M, Kato M, Takamori N, Abe M, Nishiuchi T, et al. Aggressive Antiplatelet Therapy Before Coronary Stent Implantation in Acute Coronary Syndrome with Essential Thrombocythemia: A Case Report. Angiology. 2003 Jul 1;54(4):485–90.



