Introduction
Necrotizing pneumonia (NP) is a rare and severe community-acquired pneumonia (CAP) complication, which is characterized by parenchymal consolidation, peripheral necrosis and multiple small cavitary lesions. NP belongs to a spectrum of pulmonary parenchymal destruction, lying between lung abscess and pulmonary gangrene, the final stage of lung destruction.1 Streptococcus pneumoniae is the most important etiological CAP agent, but only about 6% of pneumococcal pneumonia are necrotizing.2, 3 In fact, reviewing the literature, to our knowledge, only 78 cases of pneumococcal NP in adults are reported since 1968.1-10
In this manuscript, we describe an exuberant and severe case of CAP NP in a middle‑aged man. A brief review of this infrequent pathology is performed.
Clinical Case
We report a case of a 59-year-old construction worker with multiple cardiovascular risk factors including former smoking with chronic bronchitis and diabetes mellitus with poor metabolic control. The clinical presentation was a 1-week history of purulent productive cough, malaise and fever, with later development of dyspnea and left pleuritic chest pain. He was admitted in the emergency department with respiratory distress signs, oxygen desaturation (85% on room air) and tachycardia, but with hemodynamic stability. Lung exam findings included diminished breath sounds on the left and rhonchi bilaterally. Examination of the oral cavity revealed dental caries in the molar teeth. Arterial blood gases at room air revealed: pH 7.4, PaO2 50mmHg, ratio PaO2/FiO2 238, PCO2 30 mmHg, HCO3- 18 mmol/L, lactate 5.7 mmol/L. Analytically, an inflammatory syndrome (leucocytosis of 12.9 x 103/uL, 94% neutrophils and a C-reactive protein of 344 mg/L), an acute kidney injury (creatinine of 1.51 mg/dL) and a hyperosmolar hyperglycaemic state were documented. Chest radiogram showed an almost entire left hemithorax opacity and urinary pneumococcal antigen was positive. We assumed the diagnosis of sepsis due to pneumococcal CAP (Sequential Organ Failure Assessment Score: 3). Blood cultures were obtained and intravenous empirical antibiotics were initiated with amoxicillin/clavulanic acid and azithromycin.
Shortly upon admission, respiratory distress worsened (ratio PaO2/FiO2 121). Patient was sedated, intubated, put on mechanical ventilation and under vasopressor support. Thoracic computed tomography (CT) revealed a NP with entire left lung hepatization with multiple small cavitary lesions coalescing into a larger one in the upper left lobe (Figure 1). No parapneumonic effusion was documented. Blood cultures were positive for pan‑sensitive Streptococcus pneumoniae. The antimicrobials were switched to intravenous penicillin and clindamycin with a favourable evolution. Patient was extubated on the 8th day but, at 13th hospitalization day, a hospital‑acquired over‑infection with Klebsiella oxytoca (growth in sputum cultures) was observed. Antibiotics were adjusted based on antibiogram, replacing penicillin by ceftriaxone and clindamycin was kept. Bronchoscopy showed no macroscopic changes and the bronchoalveolar lavage cultures were negative.
The patient gradually improved, completing 6 weeks of antibiotics. CT scan was repeated, revealing smaller and less extensive cavitation area but still showing upper left lung lobe consolidation with non‑enhancing contrast areas. At discharge, patient presented with sustained apyrexia and without hypoxemia. Despite the significant imaging changes on CT scan, due to patient clinical recovery with medical treatment alone, surgical devitalized tissue debridement was not required. Patient continued a respiratory kinesiotherapy program. At 6 months, patient reported few respiratory symptoms and functional breathing tests showed moderate obstructive ventilatory disturb. He was treated with inhaled indacaterol and glycopyrronium bromide with good response. He maintains follow-up at Pneumology Consultation.
One year later, CT scan (Figure 2) showed a large cavitary lesion with air content in the left pulmonary apex, thickening of the surrounding pleuropulmonary interface, bronchiectasis and atelectasis extending to the anterior segment. Additionally, an upper left lobe and lingula volumetric reduction with ipsilateral mediastinal shift was observed.
Discussion
In CAP, the underlying lung architecture during the acute stage of infection is normally preserved whatever its severity. Unusually, progression towards necrosis and parenchymal destruction occurs. Several factors are involved in the pathogenesis of NP: overall host health and immune status; host inflammatory response; pathogen virulence and antibiotic resistance pattern; inoculum size; small and large vessels vasculitis/thrombosis development and treatment delay.4 In adults, NP develops usually in men with concomitant medical diseases that confer some immunosuppression degree (diabetes mellitus, alcoholism, smoking, chronic lung diseases, advanced age and corticosteroid therapy).9, 11 Poor dentition and medical conditions that predisposes to dysphagia/aspiration are also risk factors to NP.11 In our patient, the presence of poorly controlled diabetes, chronic obstructive pulmonary disease, poor dentition, multilobar involvement and delay in seeking medical support may all have contributed to the NP development.
Although anaerobes may be present in NP, they are not the primary etiological microorganism, unlike lung abscess. The common organisms associated with community-acquired NP are Klebsiella pneumoniae, Staphylococcus aureus and Streptococcus pneumoniae.1, 2, 9, 10 Regarding pneumococcal NP, type 3 pneumococcus strains have generally been considered as the most common organism in children and adults, even if other serotypes are also implicated.3 The capacity of type 3 pneumococcus causing necrosis is probably related to the inflammatory response to capsular polysaccharides, its ability to resist phagocytosis and toxin production leading to tissue damage even after its eradication.12 Although serotype 3 strain is included in the more recent pneumococcal vaccines (23-valent polysaccharide and 13‑valent conjugate), in a large series study, vaccination did not seem to provide protection against necrosis development.3 Our patient was not previously vaccinated and we do not know what serotype was involved (serotyping is not routinely performed in our hospital).
The NP diagnosis is based on clinical, imaging and complemented by histology. Clinical presentation includes the typical pneumonia symptoms and signs, although these patients may present severely ill with sepsis and rapid deterioration, requiring ventilation support and exhibiting septic shock within 72h of presentation.4 Chest radiogram normally underestimates the parenchymal destruction degree. Contrast‑enhanced CT is the standard modality for NP diagnosis3 – a pneumonic consolidation with multiple low contrast uptake areas suggesting necrosis and microabscesses. As the parenchymal destruction progresses, the multiple small abscesses may coalesce to larger cavities and eventually evolves to frank lobar gangrene. Radiographically, the pulmonary gangrene indicative signs are the pulmonary arterial supply obliteration to the involved segment/lobe and lack of contrast uptake with central necrosis affecting >50% of the involved lobe.1 The right upper lobe is the most often affected. Pleural involvement may manifest as pleural thickening, parapneumonic effusion or empyema.11 Other NP complications include high output bronchopleural fistula, massive hemoptysis and bilateral diffuse pneumonia.1, 9 In our patient, the diagnosis of pulmonary gangrene was not established because central pulmonary arterial supply was still patent and the necrotic area was <50% of the upper left lobe.
Establish the causative microorganism is essential to promptly direct antibiotic treatment. This includes sputum and blood cultures plus urinary antigens for Streptococcus pneumoniae and Legionella pneumophila. Tracheobronchial aspiration and bronchoalveolar lavage cultures are important in intubated patients and those who cannot produce sputum or have risk factors for unusual organisms, respectively.13 If pleural effusion coexists, cultures of the pleural fluid should be obtained.11 However, an etiological pathogen is identified only in 51%.14
The NP management requires, besides supportive care, intravenous empirical broad‑spectrum antibiotics to the commonly implicated pathogens and tailored to the local susceptibility patterns. As previously mentioned, anaerobes are not the primary inciting organism, but it is well recognized the synergistic tissue necrosing effect of a combined aerobic/anaerobic infection.12 The clinical clues that suggest pulmonary infection with anaerobes are: a putrid discharge (sputum, pleural fluid, breath); an associated condition that predisposes to aspiration; an infection in a dependent segment and late complications such as abscess formation, NP or a bronchopleural fistula underlying an empyema.15 Because anaerobes are difficult to culture, it is reasonable to treat, even if cultures are negative, particularly in patients with extensive parenchymal involvement or that are septic.10 Thereby, an initial antibiotic empirical scheme for community-acquired NP may be broad-spectrum penicillins or ceftriaxone associated with clindamycin or metronidazole.11 For allergic patients, respiratory quinolones (e.g. levofloxacin) are a viable alternative. When considering community‑acquired methicillin‑resistant Staphylococcus aureus, glycopeptides or linezolid are the choices. Antipseudomonas b‑lactams or/and quinolones or even carbapenems are initiated in hospital‑acquired NP.16
Close surveillance is required since decreased blood perfusion in the affected lung may compromise proper tissue antibiotics penetration, leading to medical treatment failure. In such cases, surgical intervention may be required to perform necrosis debridement and manage the pleural involvement.4 In empyema, chest tube for drainage (and sometimes fibrinolytic therapy), should be attempted initially. Nevertheless, patients may undergo decortication and pleural debridement in non-resolving empyema cases. For the necrotic parenchyma resection, several surgical procedures can be done, as conservative as possible - options include debridement, wedge resection, segmentectomy, lobectomy or pneumonectomy.1, 9
Managing NP patients is challenging. To the date, there are no guidelines determining the indications and optimal timing for surgery. Besides, in the absence of pulmonary gangrene, it is unknown whether surgical resection for NP is superior to medical treatment because the literature only comprises case reports and small case series that do not compare both therapeutic options.1, 4, 9, 10, 17, 18 Thus, it is important, early in the disease course, the discussion with the thoracic surgery team. Despite the radiographic exuberance and slow improvement, our patient was managed successfully with medical therapy alone without functional incapacity, regardless of the parenchymal sequelae.
Conclusion
NP is an uncommon clinical entity with a high morbimortality. Its early recognition and subsequent therapeutic optimization will improve prognosis. Physicians should consider it when the patient condition is not improving despite appropriate antibiotics. Further research is required to guide treatment and define the indications and optimal timing for pulmonary resection.
Figura I
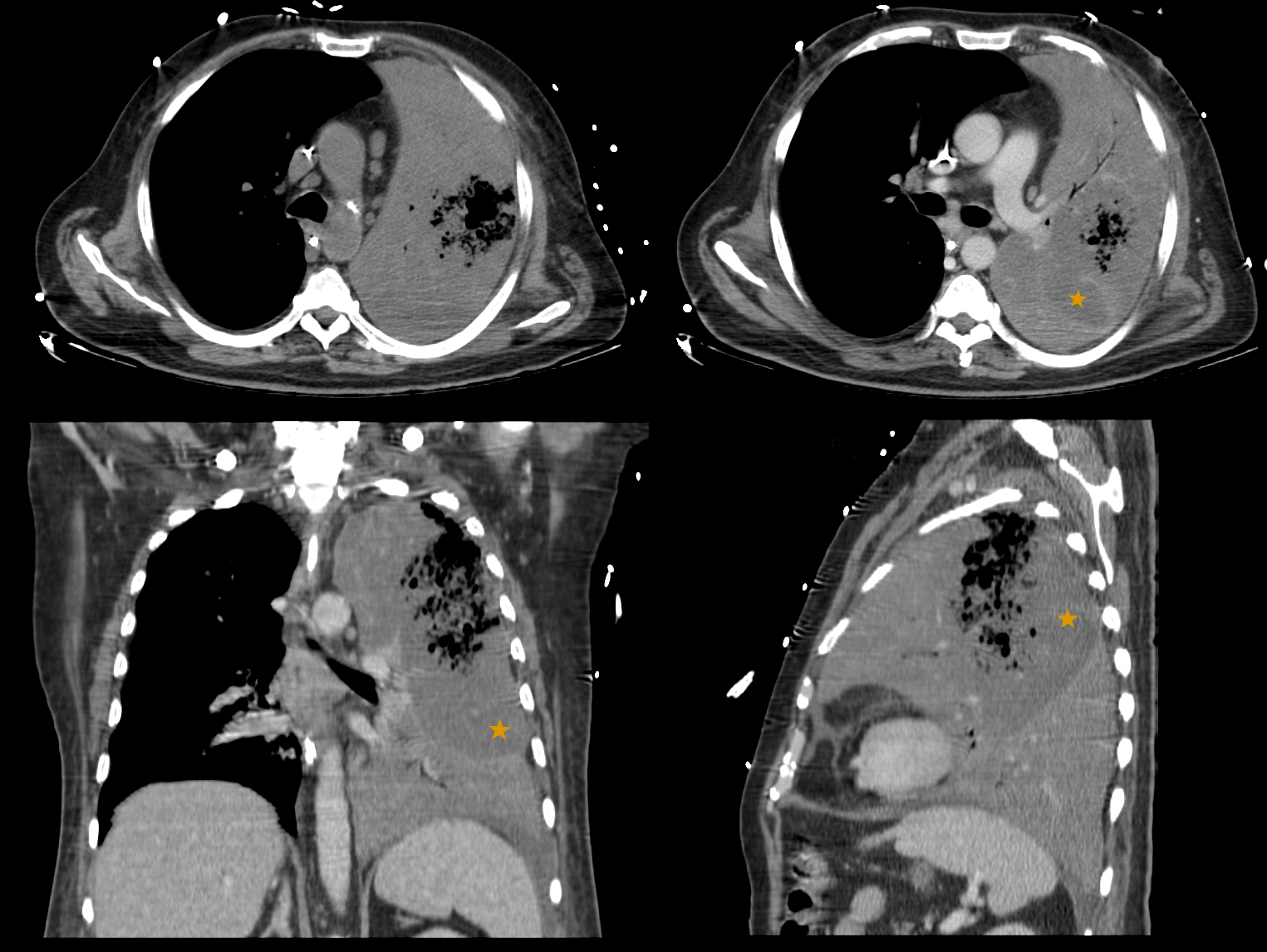
Figure 1: Initial thoracic CT scan showing pulmonary hepatization of the entire left lung, with multiple areas of low contrast uptake suggesting hypoperfusion (orange star) and multiple small cavitary lesions that coalesce into a larger one in the upper left lung lobe.
Figura II
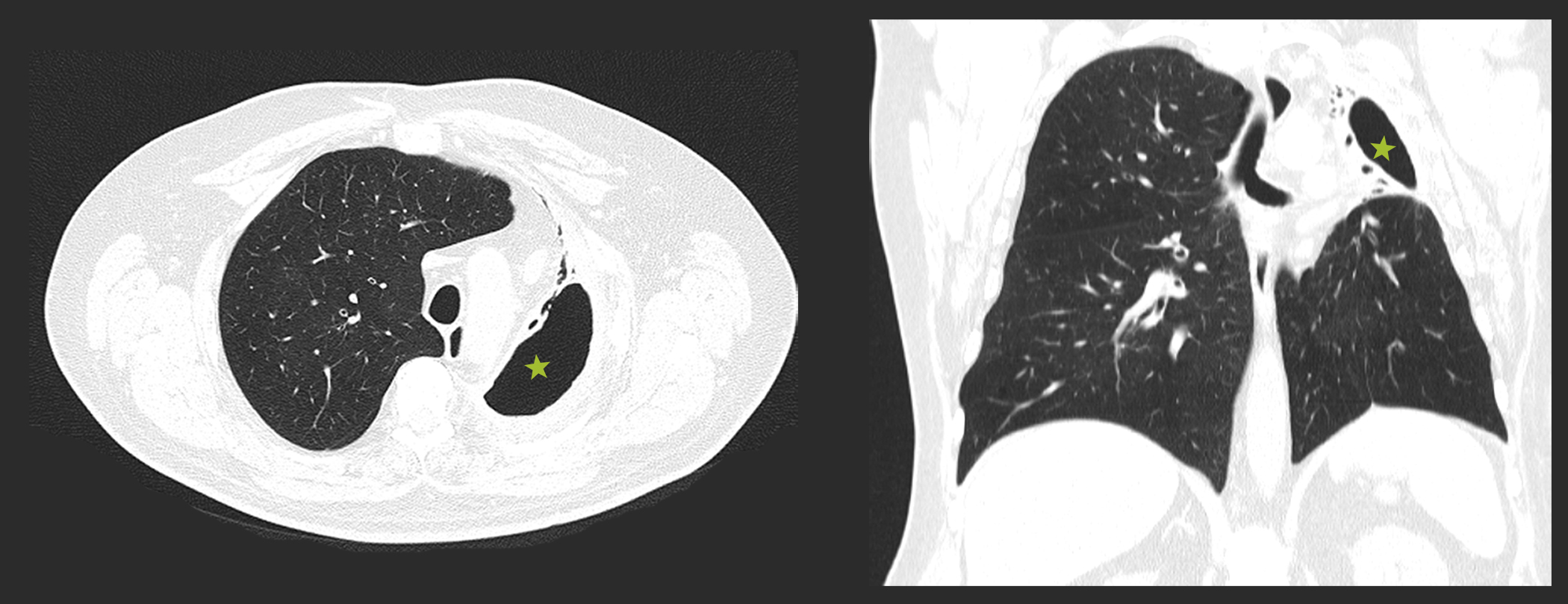
Figure 2: Thoracic CT scan repeated one year after, showing a large cavitary lesion in the left pulmonary apex (green star) and a volumetric reduction of the upper left lung lobe and lingula with ipsilateral mediastinal shift.
BIBLIOGRAFIA
1. Reimel BA, Krishnadasen B, Cuschieri J, Klein MB, Gross J, Karmy-Jones R. Surgical management of acute necrotizing lung infections. Can Respir J. 2006;13(7):369-73.10.1155/2006/760390
2. Seo H, Cha SI, Shin KM, Lim JK, Yoo SS, Lee J, et al. Clinical relevance of necrotizing change in patients with community-acquired pneumonia. Respirology. 2017;22(3):551-8.10.1111/resp.12943
3. Pande A, Nasir S, Rueda AM, Matejowsky R, Ramos J, Doshi S, et al. The incidence of necrotizing changes in adults with pneumococcal pneumonia. Clin Infect Dis. 2012;54(1):10-6.10.1093/cid/cir749
4. Chatha N, Fortin D, Bosma KJ. Management of necrotizing pneumonia and pulmonary gangrene: a case series and review of the literature. Can Respir J. 2014;21(4):239-45.10.1155/2014/864159
5. Limelette A, Guillard T, Boubee ML, Petit JS, Vernet-Garnier V, Grillon A, et al. [Necrotizing pneumonia due to Streptococcus pneumonia]. Ann Biol Clin (Paris). 2015;73(4):491-4.10.1684/abc.2015.1064
6. Norte A, Santos C, Gamboa F, Ferreira AJ, Marques A, Leite C, et al. [Necrotizing pneumonia - a rare complication]. Acta Med Port. 2012;25(1):51-5
7. Salahuddin N, Baig-Ansari N, Fatimi SH. Unusual case of non-resolving necrotizing pneumonia: A last resort measure for cure. J Pak Med Assoc. 2016;66(6):754-6
8. Seo H, Cha SI, Shin KM, Lim J, Yoo SS, Lee J, et al. Focal necrotizing pneumonia is a distinct entity from lung abscess. Respirology. 2013;18(7):1095-100.10.1111/resp.12124
9. Tsai YF, Tsai YT, Ku YH. Surgical treatment of 26 patients with necrotizing pneumonia. Eur Surg Res. 2011;47(1):13-8.10.1159/000327684
10. Karmy-Jones R, Vallières E, Harrington R. Surgical Management of Necrotizing Pneumonia. Clinical Pulmonary Medicine. 2003;10(1):17-25
11. Tzeng D, Markman M, Hardin K. Necrotizing Pneumonia and Pulmonary Gangrene: Difficulty in Diagnosis, Evaluation, and Treatment. Clinical Pulmonary Medicine. 2007;14:166-70.10.1097/01.cpm.0000263685.24879.26
12. Hammond JM, Lyddell C, Potgieter PD, Odell J. Severe pneumococcal pneumonia complicated by massive pulmonary gangrene. Chest. 1993;104(5):1610-2.10.1378/chest.104.5.1610
13. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67.10.1164/rccm.201908-1581ST
14. Bates JH, Campbell GD, Barron AL, McCracken GA, Morgan PN, Moses EB, et al. Microbial etiology of acute pneumonia in hospitalized patients. Chest. 1992;101(4):1005-12.10.1378/chest.101.4.1005
15. Bartlett JG. Anaerobic bacterial infection of the lung. Anaerobe. 2012;18(2):235-9.10.1016/j.anaerobe.2011.12.004
16. Tsai YF, Ku YH. Necrotizing pneumonia: a rare complication of pneumonia requiring special consideration. Curr Opin Pulm Med. 2012;18(3):246-52.10.1097/MCP.0b013e3283521022
17. Schweigert M, Dubecz A, Beron M, Ofner D, Stein HJ. Surgical therapy for necrotizing pneumonia and lung gangrene. Thorac Cardiovasc Surg. 2013;61(7):636-41.10.1055/s-0032-1311551
18. Schweigert M, Giraldo Ospina CF, Solymosi N, Karmy-Jones R, Dubecz A, Fernandez MJ, et al. Emergent pneumonectomy for lung gangrene: does the outcome warrant the procedure? Ann Thorac Surg. 2014;98(1):265-70.10.1016/j.athoracsur.2014.03.007

