CASE DESCRIPTION
A 66-year-old man presented to the emergency department with exertional dyspnoea, abdominal pain and dark stools, which had started two days before. He had medical history of alcoholic liver cirrhosis with portal hypertension and episodes of variceal haemorrhage. There was no history of tobacco use or chronic obstructive pulmonary disease. The patient had poor adherence to medical appointments, lacked treatment compliance and maintained alcohol consumption (about 80 grams per meal).
The physical examination revealed digital clubbing and spider nevi telangiectasis. Cyanosis and ascites were not present. The vitals were normal with exception to oxygen saturation level (77% on room air). There was no jugular vein distention or peripheral oedema.
Laboratory analysis showed macrocitosis (haemoglobin 15.3 g/dl (N 13.0-17.0) and mean corpuscular volume 109.7 fl (N 80.0-96.1)); no leucocytosis; platelet count of 71x109/L (N 150-400); normal renal function; altered liver cell integrity markers (aspartate aminotransferase 74 U/L (N<40), alanine transaminase 30 U/L (N < 41) and altered liver function tests (total bilirubin 4.71 mg/dL (N<1.40), direct bilirubin 2.28 mg/dL (N< 0.3), albumin 2.8 g/dl (N 3.5-5.2), prothrombin time 18.4 s (N<14), partial thromboplastin time 34. 5 s (N 23-38)); slightly high C-reactive protein (1.51 mg/dl (<0.50)) and D-dimers of 0.5 μg/Ml (N < 0.3 μg/ml).
At the emergency room (ER), the patient suffered two episodes of haematemesis.
Upper endoscopy was performed and showed small eradicated oesophageal varices, erosive gastritis and Forrest II gastric ulcer 2A/2B, treated with adrenaline injection and clips.
CT-pulmonary angiography performed one day after hospital admission excluded pulmonary embolism but revealed a pneumonia in the left lower lobe. Abdominal ultrasound showed a markedly heterogeneous and micronodular liver (probably due to cirrhosis) and splenomegaly with no ascites.
The patient was started on proton pump inhibitors and antibiotics and was admitted to an Internal Medicine ward. After a 10 day empiric antibiotic regime the patient remained hypoxemic and had persistent dyspnoea with low exercise tolerance and platypnea. Oxygen saturation levels improved in recumbent position; a finding consistent with orthodeoxia. This was confirmed by blood gas analysis (Table 1).
A F99_Technetium macroaggregated albumin lung scintigraphy showed presence of the radiodrug in the kidneys and spleen, a finding compatible with a right to left shunt. Transthoracic contrast echocardiography showed air bubbles in the left heart after 5 cycles, corroborating the existence of an intra-pulmonary right-to-left shunt and therefore making the diagnosis of hepatopulmonary syndrome. (Figure 1)
The patient was discharged on supplemental oxygen with a 31% venturi mask. He was started on garlic tablets - allium sativum (1,8gr/m2 Body Surface Area) and was strongly advised to maintain alcoholic abstinence. The patient is asyntomatic, although no improvement on oxygenation has been achieved thus far.
The patient was referred to a liver transplantation centre and is in the waiting list for an elective liver transplant.
DISCUSSION
Hepatopulmonary syndrome (HPS) consists of a triad of hepatic dysfunction, hypoxemia and extreme vasodilatation. It is characterized by a defect in oxygenation induced by pulmonary vascular dilation in the setting of liver disease.
1,2 It was first described in 1884 by Flückiger based on the observation of a woman with cyanosis, clubbing and cirrhosis.
3The estimated prevalence of this entity is 15% in cirrhotic patients.3 It occurs in 10 to 30% of patients with liver disease referred for liver transplantation.4 HPS is typically related to cirrhosis or portal hypertension but it may also develop in the setting of acute hepatitis, chronic liver disease without portal hypertension and in patients with vascular abnormalities limiting the hepatic venous outflow to the lung. 3,5,6
The pathogenic hallmark is microvascular dilation of pulmonary precapillary and capillary vessels which is thought to be partially related to nitric oxide mediated pulmonary vasodilation.2,5,6 This leads to increased pulmonary blood flow and cardiac output causing a perfusion ventilation mismatch and anatomical and functional right-to-left shunting. In addition, decreased intrapulmonary blood transit time in the setting of hyperdynamic circulation and increased alveolar capillary diameter leads to impaired lung diffusion.2,5,6
Dyspnoea, particularly on exertion, albeit nonspecific, is the predominant presenting symptom. In a patient with liver disease, the presence of digital clubbing, cyanosis and severe hypoxemia (partial pressure of oxygen < 60mmHg), in the absence of associated cardiopulmonary disease, strongly suggests HPS.2,6 Platypnea (dyspnoea exacerbated when sitting up and improving when lying down) and orthodeoxia (decrease in partial pressure of oxygen when changing from lying down to sitting up) are classically related to this syndrome. This phenomenon is explained by the worsening of diffusion-perfusion matching and an increase of shunt fraction in the upright position due to blood flow redistribution with increased perfusion of lower lobes.2,6,7
The diagnosis of HPS depends on demonstration of hypoxemia and pulmonary vascular dilatations on a patient with liver disease. An arterial blood gas sample in the sitting position should be obtained to determine hypoxemia: recommended cut-off values are partial pressure of oxygen < 80mmHg (< 70mmHg if age > 64 years old) or alveolar-arterial oxygen gradient (AaO2) ≥ 15mmHg (≥ 20mmHg if age > 64 years old).6,7 The chest radiograph is frequently nonspecific. Pulmonary function testing may show a low diffusing capacity for carbon dioxide suggesting structural remodelling of pulmonary vasculature.2
Transthoracic contrast echocardiography with shaken saline solution (to produce microbubbles) is a sensitive and non-invasive method for screening vascular pulmonary dilatations and is now considered the gold standard.6 Under physiological circumstances, once microbubbles leave the right atrium, they become trapped in the pulmonary capillaries and cannot be visualized in the left heart. In HPS, bubbles can be seen in the left cavities since they are able to pass through the dilated vessels. This would happen between the third and sixth cardiac cycle after intravenous injection. An immediate appearance of echo contrast material in the left atrium suggests the presence of an intracardiac shunt.2,3,7 99_Technetium macroaggregated albumin (MAA) perfusion lung scanning, despite being able to quantify the degree of intrapulmonary shunting, cannot differentiate intracardiac from intrapulmonary shunting.3,6 Pulmonary angiography is reserved for patients with poor response to oxygen: it can identify focal arteriovenous malformations that may be embolized.8
Untreated HPS has a 5-year-survival rate of 23%.5 Survival is worse among patients with a partial pressure of oxygen of less than 50mmHg at diagnosis. Causes of death among these patients are multifactorial and generally related to progression or complications of the hepatic disease – death due to severe hypoxemic respiratory failure is rare.2
Orthotopic liver transplantation is the only curative therapy for HPS: it can improve oxygenation and pulmonary vascular dilatation with disappearance of signs and symptoms of HPS in more than 80% of transplanted patients.1,4
Supplemental oxygen should be administered to correct hypoxemia. Other medical therapies have not shown proven benefits – the therapeutic goal of reversing pulmonary vascular dilatation has not been achieved. Oral garlic supplementation has shown some benefit in improving oxygenation in one pilot study, but the underlying mechanisms have not been elucidated yet.5,6 Inhaled nitric oxide, pentoxifylline and methylene blue – molecules that act by blocking vasodilation or angiogenesis – have been used without benefits.7 Other strategies targeting portal hypertension, namely nitrates, β-blockers or transjugular portosystemic shunting exert no beneficial effect on oxygenation in this subgroup of cirrhotic patients.7
In the reported case HPS was suspected due to platypnea and demonstration of orthodeoxia. The diagnosis was confirmed by transthoracic contrast echocardiography, which detected microbubbles in the left heart after five cycles. Severe hypoxemia was well tolerated by the patient, probably due to its chronicity and the underlying hyperdynamic status (increased heart rate, cardiac output and plasma volume with reduced vascular resistance and blood pressure).
The hepatopulmonary syndrome is a severe disease with a low five-year survival rate that only resolves with a liver transplant. Considering that the patient is young, otherwise healthy and did not have any alcohol consumption in the last 7 months, he was considered a suitable candidate for transplantation and is currently in the transplant list.
Quadro I
Blood gas analysis in dorsal decubitus and supine position confirming orthodeoxia.
| ABG parameters | Room air | Dorsal decubitus position (FiO2 31%) | Supine position (FiO2 31%) | Normal range |
| | | | | |
| PaCO2 (mmHg) | 32 | 28 | 29 | 35.0-45.0 |
| PaO2 (mmHg) | 45 | 119 | 67 | 75.0-100.0 |
| SpO2 (%) | 74 | 100 | 89,5 | 92.0-98.5 |
| | | | | |
Figura I
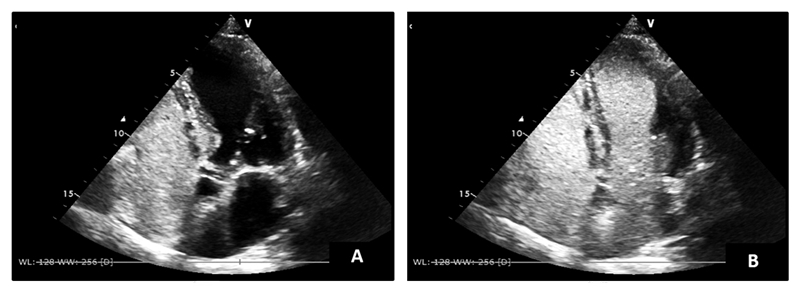
Transthoracic contrast echocardiography with initial microbubbles opacifying the right heart chambers (A) and after 5 cycles appearing on the left heart chambers (B).
BIBLIOGRAFIA
1. Ninaber MK, B de Vaal J, Corsmit O et al. Severe Arterial Hypoxemia in Liver Cirrhosis. Respiratory Care 2009; 54 (3): 393-7.
2. Rodríguez-Rosin R, Krowka MJ. Hepatopulmonary Syndrome – A Liver-Induced Lung Vascular Disorder. N Engl J Med 2008; 358: 2378-1387.
3. Grilo-Bensusan I, Pascasio-Acevedo JM. Hepatopulmonary syndrome: What we know and what we would like to know. World J Gastroenterol 2016; 22(25): 5728-41.
4. Bauer M, Fuhrmann V, Wendon J. Pulmonary complications in liver disease. Intensive Care Med; https://doi.org/10.1007/s00134-019-05721-y.
5. Qadir N, Wang T, Barjaktarevic I et al. Acute Respiratory Failure and Pulmonary Complications in End-Stage Liver Disease. Semin Respir Crit Care Med 2018; 39: 546-55.
6. Lv Y, Fan D. Hepatopulmonary Syndrome. Dig Dis Sci 2015; https://doi.org/10.1007/s10620-015-3593-0.
7. Hoeper MM, Krowka MJ, Strassburg CP, Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004; 363: 1461-68.
8. Aldenkortt F, Aldenkortt M, Caviezel L et al. Portopulmonary hypertension and hepatopulmonary syndrome. World J Gastroenterol 2014; 20(25): 8072-8081.


