INTRODUCTION
Good’s Syndrome specifies the association between the presence of thymoma and hypogammaglobulinemia and was first recognized by Dr Robert Good in 1954. Frequently manifests with recurrent infections, in the 4th and 5th decades of life and 6 to 11% of the patients with thymoma present with hypogammaglobulinaemia. The cause and pathogenesis are unknown, though the basic defect may be in the bone marrow (pre-B cell arrest, impaired maturation of erythroid and myeloid precursors).1,2
Thymomas appear from thymic epithelial cells with an unspecified etiology. They occur with no genders predominance and the majority presented in this syndrome are benign, although they can express benign or malignant behaviour.3
Good’s syndrome is a complex immunodeficiency characterized by a variable phenotype. Reviews suggest that the course of the disease is not well understood and it carries a relatively poor prognosis with a mortality of 44.5 to 57%.4
CLINICAL CASE
We present the case of a 64 year old woman, non-smoker, with several non-severe respiratory infections (sputum and blood cultures were negative) in a period of 3 years that needed ambulatory antibiotic treatment. Thoracic-CT (Fig. 1) showed a left nodular lesion with 10 cm of diameter, that was biopsied revealing a thymoma.
During hospitalization, she remained asymptomatic, apyretic, hemodynamically stable, with normal pulmonary auscultation, without suplemental oxygen and completed 2 blood units without complications.
The analytic evaluation uncovered a normocytic normochromic anemia (Hb 5.7 g/dL). Haptoglobin, Coombs direct and indirect test were negative. From the remaining laboratory study: medulogram with pure red cell aplasia, hyperplasia of the lymphoid series, immunophenotyping with 70% lymphoid population, increased T cell markers, absence of B cell markers (CD 19 and CD 20 0%); autoimmunity (Anti-Nuclear, Anti-dsDNA, Anti-Nucleossomes, Anti-Smooth Muscle and Anti-Ribossomes antibodies) remained negative, hypogammaglobulinaemia (IgG 198 mg/dL, IgA <24mg/dL, IgM <17.5mg/dL). Viral serologies (Immunodeficiency virus, Cytomegalovirus, B and C hepatitis), tumor markers, cultures and parasitological feces exams were negative. Upper digestive endoscopy and colonoscopy were normal and thoracic-abdominal-pelvic-CT didn’t reveal any other lesions.
The patient initiated 600 mg/kg/dose infusion of human intravenous immunoglobulin for 48 hours. After normalization of the values of IgG (IgG>600 mg/dL) she was submitted to surgery, with removal of the mediastinical mass. To this date, the patient continues with IgG levels within normal limit, the number of respiratory infections and antibiotic use has decreased, but still needs continuing blood transfusions to treat the anaemia.
DISCUSSION
Good’s syndrome is a rare cause of combined T and B cell immunodeficiency in adults and the immunologic defects may be present before or after identification of the thymoma. Patients have a significant morbidity relating with autoimmune complications and increased susceptibility to infections, thus the most frequent presentations are recurrent pulmonary and sinusitis infections.5
Proposed mechanisms include impaired haemopoiesis from thymoma paraneoplastic phenomena and lack of interferon-like cytokines from thymic T cells to promote B cell growth and differentiation. Although thymectomy is indicated, it does not always reverse the immunodeficiency and ongoing IVIg may be required, as it occurred with our patient.6
Hematological disorders are frequently present. Half of the patients have anaemia and a low white blood cell count. There have been described monoclonal gammopathies, T cell tumors, aplastic, haemolityc and pernicious anaemia. Pure red cell aplasia is the most common autoimune presentation of Good’s syndrome, manifests with a normochronic and normocytic anemia and is present in this patient. Some reports noticed that 20% of patients have thrombocytopenia, 18% neutropenia and eosinophils are absent.7,8
The most typical microorganisms associated with infection in patients with Good’s syndrome are encapsulated bacterial such as Haemophilus influenzae (24%) and Streptococcus pneumoniae (8%). Gram negatives such as Pseudomonas and Klebsiella were found in patients with bronchiectasis and Giardia, Salmonella and Campylobacter jejuni in those with chronic diarrhea. Viral infections were current in 40% (CMV the most regular), while systemic fungal infections are not a feature. In our patient, microbiological, viral, fungal and feces cultures and parasitological exams were negative.9,10
There are commonly paraneoplastic syndromes triggered by the thymoma and autoimmune disorders like pure red cell aplasia. Myasthenia gravis occurs in 45% of cases, so is the most recurrent. Other conditions associated are diabetes mellitus, systemic lupus erythematous, rheumatoid arthritis, thyroiditis, Sjogren syndrome and ulcerative colitis.11
Overall, in 80% of patients with thymoma, an anterior mediastinal mass is notable on a postero-anterior chest x ray (Fig. 2). In few cases, an accidentally discovery in an assymptomatic patient is the first presentation, as it was described in our patient. Hence, a computed tomography scan of the chest should be requested because it can define the extent of the mass before the surgery.11 Treatment is based on removal of the mass and immunoglobulin replacement treatment, that can help reduce in the numbers of bacterial sinopulmonary infections. Other therapeutic such as immunossupressors, plasmapheresis, splenectomy had scarcer results. 12
Prognostic data are limited; nonetheless, a single-center report cites 70% to 33% survival at 5 and 10 years, respectively. 13 The most important indicator of long term prognosis is completeness of tumour ressection, as well as prevention of invasive growth and local symptoms, however it does not cure. In the presence of paraneoplastic conditions, the extent of infectious problems, which is associated by the level of B and T cell dysfunction, is going to determine the prognosis. Association with thymoma and myasthenia gravis is a factor of enhanced outcome since an earlier identification of the mass and decreases the rate of recurrence. The main causes of death are as a consequence of infection, autoimmune disease or hematological complications.14
Good’s syndrome should be considered in patients over 40 years of age with recurrent respiratory infections and unexplained antibody deficiency. We describe this rare case thus physicians should be alert of this condition and measure the serum level of immunoglobulin in any suspicious case of thymoma and repeated infection. Early screening could prevent severe mortality and morbidity, so it is important to increase clinical awareness about the immunological and clinical profile of this syndrome. Further studies are needed to clarify this clinical entity. 15
Figura I
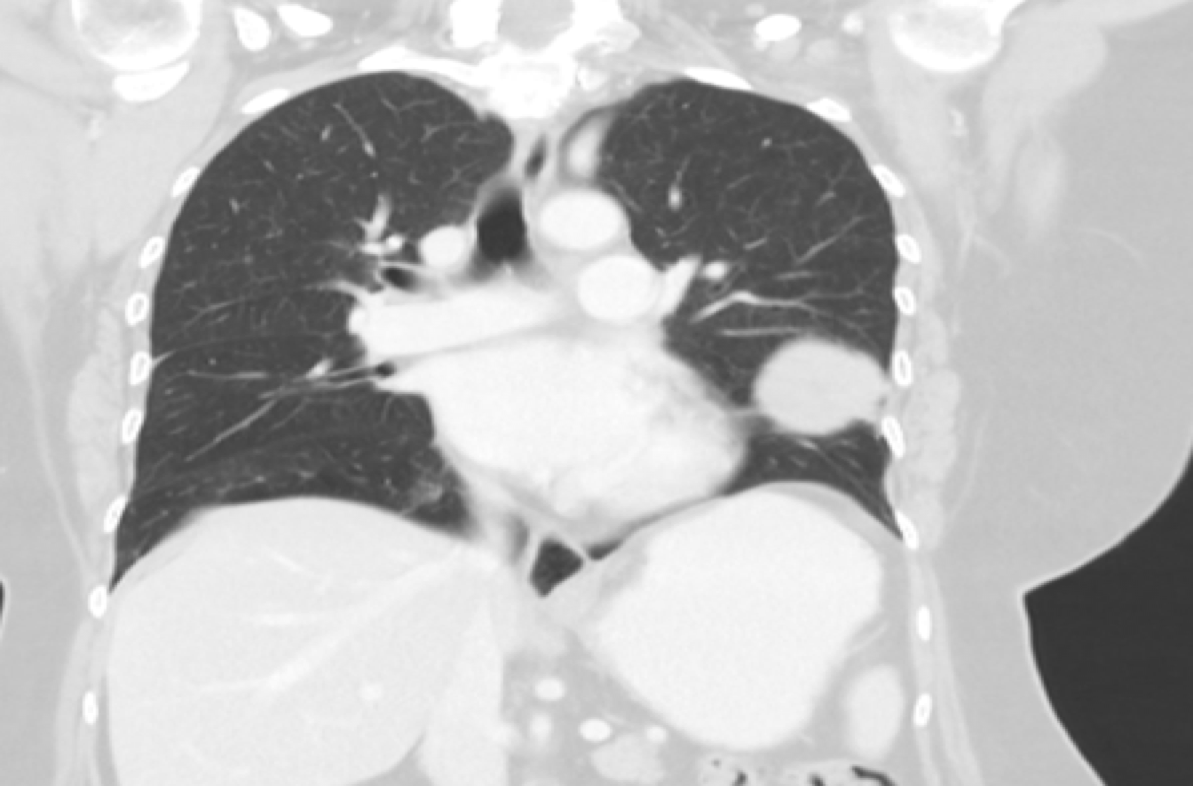
Thoracic CT pressenting a mass located in the base of the left lung
Figura II
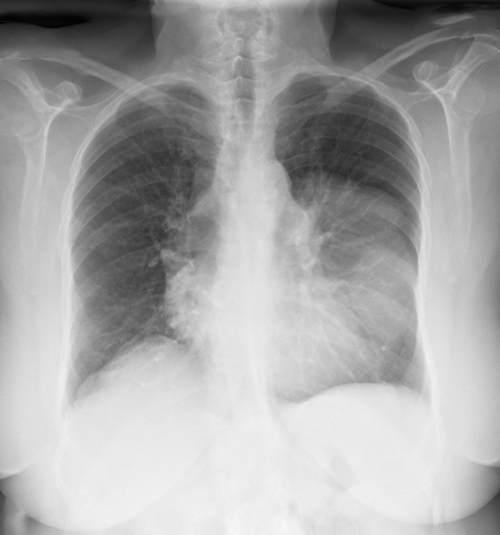
Chest X-ray showing a diffuse hypotransparency occupying the lower two thirds of the left lung
BIBLIOGRAFIA
REFERENCES
1. Kelesidis T, Yang O. “Good’s syndrome remains a mystery after 55 years: A systematic review of the scientific evidence.” Clin Immunol 2010; 135: 347‑63.
2. Kelleher P, Misbah SA. “What is Good’s syndrome? Immunological abnormalities in patients with thymoma.” J Clin Pathol 2003; 56: 12‑6.
3. Joven MH, Palalay MP, Sonido CY. “Case Report and Literature Review on Good´s Syndrome, a Form of Acquired Immunodeficiency Associated with Thymomas.” Hawaii J Med Public Health 2013; 72(2): 56-62.
4. J.-P. Dong, W. Gao, G.-G. Teng, Y. Tian, and H.-H. Wang, “Characteristics of goodʼs syndrome in China,” Chinese Medical Journal, vol. 130, no. 13, pp. 1604–09, 2017.
5. K. Akinosoglou, M. Melachrinou, D. Siagris et al., “Good’s syndrome and pure white cell aplasia complicated by cryptococcus infection: a case report and review of the literature,” Journal of Clinical Immunology, vol. 34, no. 3, pp. 283–8, 2014.
6. Z. Fumeaux, P. Beris, B. Borisch et al., “Complete remission of pure white cell aplasia associated with thymoma, autoimmune thyroiditis and type 1 diabetes,” European Journal of Haematology, vol. 70, no. 3, pp. 186–9, 2003.
7. M. Cheng and M. S. Anderson, “Thymic tolerance as a key brake on autoimmunity,” Nature Immunology, vol. 19, no. 7, pp. 659–64, 2018.
8. S. Raschal, J. Siegel, J. Huml, and G. Richmond, “Hypogammaglobulinemia and anemia 18 years after thymoma resection,” Journal of Allergy and Clinical Immunology, vol. 100, no. 6, pp. 846–8, 1997.
9. M. R. Khawaja, R. P. Nelson Jr., N. Miller et al., “Immunemediated diseases and immunodeficiencies associated with thymic epithelial neoplasms,” Journal of Clinical Immunology, vol. 32, no. 3, pp. 430–7, 2012.
10. L. Degos, A. Faille, M. Housset, L. Boumsell, C. Rabian, and T. Parames, “Syndrome of neutrophil agranulocytosis, hypogammaglobulinemia, and thymoma,” Blood, vol. 60, no. 4, pp. 968–72, 1982.
11. Agarwal S, Cunningham-Rundles C. “Thymoma and immunodeficiency (Good syndrome): a report of 2 unusual cases and review of the literature.” Ann Allergy Asthma Immunol 2007; 98(2): 185-90.
12. Tarr PE, Sneller MC, Mechanic LJ, Economides A, Eger CM, Strober W, et al. “Infections in patients with immunodeficiency with thymoma (Good syndrome): report of 5 cases and review of the literature.” Medicine 2001; 80(2):123-33.
13. Hermaszewski RA, Webster A.D. “Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications.” Q J Med 1993; 86: 31-42.
14. Taniguchi T, Usami N, Kawaguchi K, Yokoi K. “Good syndrome accompanied by pure red cell aplasia.” Interact Cardiovasc Thorac Surg 2009; 9(4): 750-2.
15. A. Jansen, M. van Deuren, J. Miller et al., “Prognosis of good syndrome: mortality and morbidity of thymoma associated immunodeficiency in perspective,” Clinical Immunology, vol. 171, pp. 12–17, 2016.



