Introduction: Heart failure (HF) is a clinical syndrome of major importance in adult medicine. In contrast, HF among the young is rare, but may exert a greater impact on active and income-generating individuals. Most HF-studies are based on the elderly population, and little is known about the aetiology, incidence and trends in HF among younger patients.1
Case Description: A 24 year-old male patient of african descent was brought into the Emergency Room complaining of shortness of breath at ever lower effort levels, a cough containing mucoid sputum, and edema of the lower limbs — symptoms that had been steadily worsening over the two previous weeks. More recently, he had also noticed an increase in abdominal volume. The patient denied having a fever, palpitations or dizziness, chest pain, paroxysmal nocturnal dyspnea or orthopnea, or reduced diuresis.Initial assessment revealed that the patient didn’t have a fever, was normotensive but tachycardic (HR: 150 bpm); he had no signs of respiratory distress, and showed good peripheral oxygen saturation. Venous jugular turgescence was present even at 90º. Cardiac auscultation confirmed tachycardic and arrythmic heart sounds. Pulmonary auscultation identified diminished breathing sounds in the inferior half of the right chest. Moderate ascites and edema of the lower limbs were also identified.The patient’s personal history included recently diagnosed Human Immunodeficiency Virus (HIV) and Hepatitis B Virus coinfection, which was being treated with antiretroviral drugs (Tenofovir/Emtricitabine and Darunavir/Ritonavir) (Table 1). He denied any alcoholic, tobacco or drug abuse, or any known family history of heart disease. In view of the clinical evaluation of the patient and initial laboratory study (Table 2) that revealed a B-type Natriuretic Peptide (BNP) value of 635.7 pg/mL, Inaugural Acute Heart Failure was diagnosed.
Initial electrocardiography (EKG) revealed an Atrial Fibrillation with rapid ventricular response. As the time of onset was unknown, the heart rate was controlled with digoxin and beta-blockers. Later during the patient’s stay, a second ECG showed that there had been spontaneous cardioversion to sinus rhythm, but it also revealed a right heart axis deviation and negative T-waves in the precordial V1, V2 and V3 right deviations. A chest x-ray identified a moderate right-sided pleural effusion (Figure 1), with no other significant alterations. A diagnostic thoracocentesis was performed, making it possible to characterize the pleural fluid as an exudate (according to Light’s criteria). Further study of the sample was negative for tumoral cells and cultural analysis (including Mycobacterium). Transthoracic Cardiac Ultrasound revealed severe dilation of the right chambers of the heart and right ventricular disfunction with left deviation of the interventricular septum; left chambers of the heart of normal size; slight decrease in left ventricular ejection fraction (42%); and free tricuspid valve regurgitation, which made it impossible to estimate the PSAP value although there were indirect signs of severe pulmonary hypertension. Given the patient’s personal history of HIV infection and the results obtained from the cardiac ultrasound, HIV-associated Pulmonary Hypertension was our main diagnostic hypothesis. Therefore, the patient was transferred to an experienced Pulmonary Hypertension Hospital Centre where right heart catheterization was performed and rejected this diagnosis. To discover the aetiology of the HF, the patient underwent a cardiac MRI which produced findings compatible with a major criterion for the diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC): Global hypokinesia of the free wall of the right ventricle, with an area of dyskinesia in the inferior basal region. Severe decrease in the systolic function of the right ventricle (23%). In view of these results, we concluded that the patient had a “false pulmonary hypertension” and according to the ARVC diagnostic criteria published by the International Task Force Criteria for the Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy, this patient displays two Major Criteria2 (Global or regional dysfunction and structural alteration on MRI and Repolarization abnormalities on ECG) allowing a definitive diagnosis of ARVC.
Conclusion: While ischaemic heart disease, valve disease and hypertension are predominant causes of HF among older patients, the causes of HF in the young adult are more varied. In this age group, congenital heart and inherited heart diseases and cardiomyopathies should be considered as possible causes.1
In this case HIV infection is also an important factor to consider as it is an established risk factor for pulmonary hypertension and as this disease progresses with right ventricular involvement, patients may report symptoms of heart failure. The diagnosis of HIV-associated pulmonary hypertension is one of exclusion, and secondary causes should be ruled out. Right heart catheterization is the gold standard diagnostic test for pulmonary hypertension, which is defined by a mean pulmonary artery pressure greater than 25 mmHg at rest in the setting of a normal pulmonary capillary wedge pressure. 3
ARVC is an inherited heart-muscle disorder that predominantly affects the right ventricle, in which the progressive replacement of the myocardium by fibrofatty tissue is the hallmark of the disease.4 The prevalence of ARVC is estimated to affect 1 in 5.000 of the population, and it typically appears in 10-30 year-old individuals,4 usually with symptoms such as palpitations or effort-induced syncope. However, the majority of patients are asymptomatic, and the disease is suspected by electrocardiographic and cardiac ultrasound alterations or by the identification of ventricular arrythmias.5 This cardiomyopathy is frequently underdiagnosed.5
Treatment and management of ARCV begins with risk stratification, as natural history of the disease is related to ventricular electric instability, arrhythmias, and sudden death especially in young adults. A definite risk algorithm has not been established; however risk stratification includes inquiring for the presence of symptoms of heart failure, syncope, history of malignant arrhythmic events and the existence of right ventricle dilation or dysfunction, left ventricle or both in heart ultrasound or cardiac magnetic resonance.6
In our case, our patient will undergo a lifelong clinical follow-up to periodically evaluate worsening of symptoms or progression of morphological or functional ventricular abnormalities or arrhythmias. A therapeutic plan was built for our patient, including lifestyle changes and pharmacological treatment (b-blockers and heart failure therapy). Further study is being planned in order to assess the need for ICD implantation. 6
Quadro I
HIV and HBV coinfection
| | At diagnosis | 8 months later (on treatment) |
| | | |
| Viral Load HIV-1 | 139 745 copies/mL | 2 136 copies/mL |
| CD4 T Limphocyte | 229/m3 (13.3%) | 223/m3 (10.1%) |
| CD4/CD8 ratio | 0.25 | 0.16 |
| | | |
| HBs Antigen | positive | |
| HBV antibody | positive | |
| HBe Antigen | positive | |
| DNA HBV | 170 000 000 IU/mL | 451 000 IU/mL |
| | No resistance mutations identified | |
Quadro II
Initial Laboratory Study
| | |
| | |
| White Blood Cells | 5.40 x10^9/L |
| Neutrophils: | 45% |
| Hemoglobin | 14.2 g/dL (14.0-18.0) |
| Mean Corpuscular Volume | 86.2 fL (80.0-95.0) |
| Mean Hematocrit Concentration | 28.6 pg (23.0-32.0) |
| Prothrombin time | 20.7 s |
| Activated Partial Thromboplastin Time | 42% |
| INR | 1.73 |
| Platelets | 172 x10^9/L (150-140) |
| Sodium | 132 mEq/L (136-145) |
| Potassium | 5.3 mEq/L (3.5-4.5) |
| Creatinine | 1,1 mg/dL (0.6-1.3) |
| Urea | 43 mg/dL (14-42) |
| Aspartate transaminase (AST) | 82 UI/L (4-43) |
| Alanine transaminase (ALT) | 44 UI/L (4-43) |
| Gamma-glutamyl transferase (GGT) | 133 UI/L (7-49) |
| Alkaline phosphatase | 93 UI/L (25-100) |
| Lactate dehydrogenase | 803 UI/L (200-480) |
| Total Bilirubin | 2.3 mg/dL (0.3-1.2) |
| Direct Bilirubin | 1.54 mg/dL (0.1-0.5) |
| Total Protein | 9.9 g/dL (6.6-8.7) |
| Albumin | 3.2 g/dL (3.5-5.0) |
| B-type Natriuretic Peptide (BNP) | 635.7 pg/mL |
| C-reactive protein (CRP) | 2.15 mg/dL (<0,5) |
| Procalcitonin | .05 ng/mL (0.01-0.64) |
Figura I
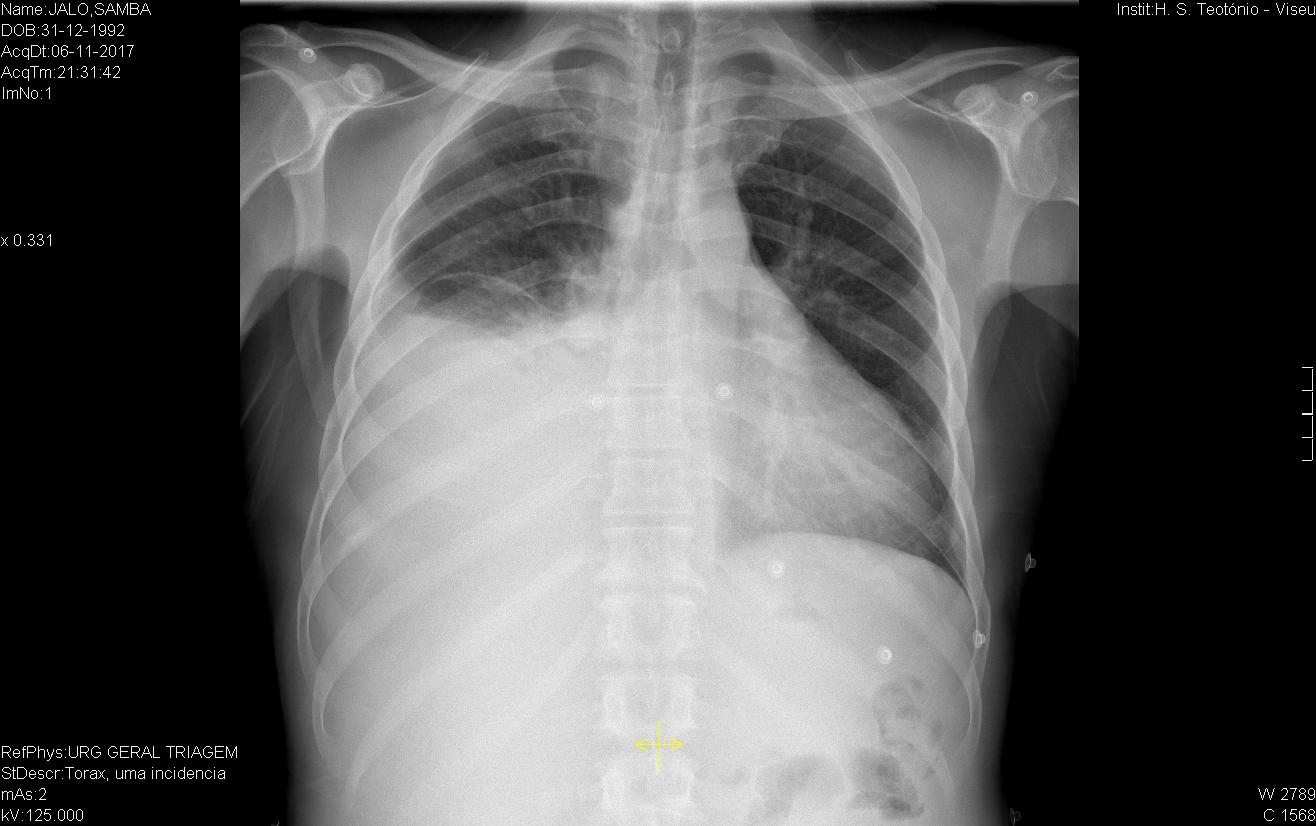
Chest X-ray showing a moderate right-sided pleural effusion.
Figura II
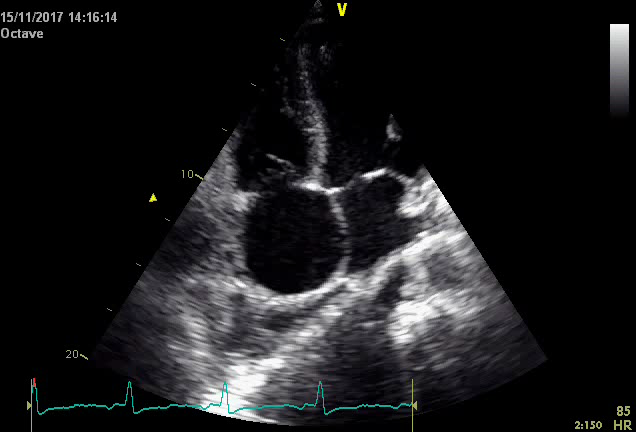
Transthoracic cardiac utlrasound revealing severe dilation of the right chambers of the heart and left deviation of the interventricular septum.
Figura III
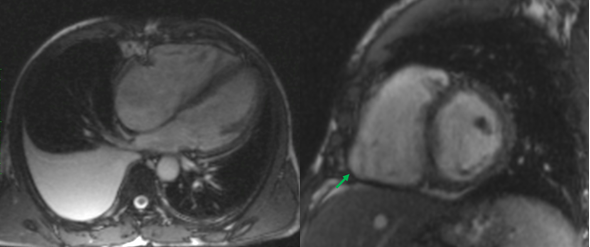
Cardiac MRI.
BIBLIOGRAFIA
1. Barasa A, Schaufelberger M, Lappas G, et al. Heart failure in young adults: 20‐year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J. 2013; 35: 25–32.
2. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010; 31: 806-14.
3. Almodovar S, Hsue P Y, Morelli J, Huang L, et al. Pathogenesis of HIV-Associated Pulmonary Hypertension. Proc Am Thorac Soc. 2011; 8(3): 308-312.
4. Corrado D, Link MS, Calkins H. Arrhythmogenic Right Ventricular Cardiomyopathy. N Engl J Med. 2017; 376: 1489–90.
5. Moreira D, Delgado A, Marlmelo B, Correia E, et al. Arrythmogenic right ventricular cardiomyopathy: Contribution of different electrocardiographic techniques. Rev Port Cardiol. 2014; 33(4): 243.e7
6. Corraro D, Wichter T, Link M S, Hauer R N W, et al. Treatment of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia - An International Task Force Consensus Statement. Circulation. 2015; 4;132(5):441-53




