Hyponatremia is a highly prevalent electrolyte disturbance, affecting approximately 28% of acute hospital care patients.1 It can occur in the setting of the syndrome of inappropriate antidiuretic hormone secretion (SIADH), characterized by euvolemic hyponatremia, low serum osmolality with elevated urine osmolality and natriuresis, without thyroid/adrenal disturbances or diuretic medications.2
Many systemic diseases have been linked to SIADH, such as lung, gastrointestinal or genitourinary malignancies, pulmonary infections and other disorders, central nervous system bleeding and tumours and certain medications.2 However, its association with Rheumatoid Arthritis (RA) is not well established.3 Interleukin-6 (IL-6) is known to play a key role in RA4 and there is increasing evidence that it may influence the non-osmotic release of vasopressin in the setting of inflammatory diseases, causing hyponatremia.5
We report a case of hyponatremia whose extensive workup led to the diagnosis of RA.
Case report
An 86-year-old Caucasian woman, previously well and independent, presented to the Emergency Department with complaints of malaise, fatigue, orthopnoea and discrete oedema on both hands and ankles for six months, worsening in the last month. She also had anorexia, dyspepsia and weight loss (10kg in the past six months). There were no palpitations, chest pain, urinary or other gastrointestinal symptoms. She had hypertension, dyslipidaemia and chronic anaemia without prior investigation. Her daily medication included carvedilol, perindopril, valsartan + hydrochlorothiazide and simvastatin.
On observation, she was pale, hydrated, oriented to person and place, but not to time and a brief neurologic examination did not reveal other changes. She was haemodynamically stable, eupnoeic with discrete bibasilar crackles in the pulmonary auscultation and febrile (tympanic temperature: 38.4ºC). She had slight oedema on both hands with painful joint palpation, local hyperaemia on the metacarpophalangeal and proximal interphalangeal joints and bimalleolar oedema.
The initial blood tests (Table 1) revealed a hypochromic microcytic anaemia, C-reactive protein (CRP) of 68mg/L without leucocytosis, normal kidney function, hyponatremia of 124mEq/L with no other electrolyte imbalances and brain natriuretic peptide (BNP) of 293pg/mL.The electrocardiogram was normal; chest radiograph showed bilateral reticular pattern.
On admission to our ward, her medication was discontinued and further workup was started. The calculated plasma osmolality was decreased (265mOsmol/kg). The absence of jugular engorgement, a very modest BNP elevation and a transthoracic echocardiogram without changes suggestive of heart failure made a hypervolemic cardiac cause for her hyponatremia less likely. Her peripheral oedema seemed to be caused by hypoalbuminemia instead of water retention. The patient was considered euvolemic and a 24h urine analysis was requested, with: increased osmolality of 510mOsmol/kg, high sodium levels of 69mEq/L, potassium 41.4mEq/L, glucose <1.0 mg/dL, urea 17.3 g/L and creatinine 602mg/24h. Her thyroid and adrenal functions were normal, her medication was reviewed and considered innocuous in this case. A diagnosis of SIADH was therefore considered the most likely cause for this hyponatremia. The patient’s complaints of weight loss, anorexia and chronic anaemia prompted investigation of a possible neoplastic cause.
We performed a thoraco-abdominopelvic contrast-enhanced computed tomography (Fig. 1) revealing mild reticulation in both lungs, traction bronchiolectasis and honeycombing, a discrete bilateral pleural effusion, hepatomegaly with regular boundaries and no parenchymal suspected lesions and a distended stomach with diffuse parietal thickening on the pre-pyloric region. It also described a diffuse irregularity on the cortical region of the right humeral diaphysis suggestive of an infiltrative tumoral lesion. Taking these alterations into account, she underwent endoscopic evaluation. Upper endoscopy revealed superficial gastropathy positive for Helicobacter pyloriand colonoscopy a single 2mm sessile rectal polyp. A brain magnetic resonance imaging showed signs of chronic ischemic microangiopathic leukoencephalopathy and a non-recent area of lacunar infarction on the left corona radiata; no other relevant changes were found, namely on the pituitary gland. The HIV, HBV and HCV screenings, VDRL test and IGRA were all negative. Blood and urine cultures were negative. Protein electrophoresis had total proteins of 62.5g/L with reduced albumin/globulin fraction (0.6) and bisalbuminaemia, elevated beta-2-globulins (13.7%) and gamma-globulins (22.2%). The patient also had an elevated beta-2-microglobulin (6.89 mg/L). Immunofixation electrophoresis revealed a slight polyclonal elevation of all immunoglobulins.
The whole-body bone scintigraphy (Fig. 2) showed generalized hypercaptation of the isotope by the joints on both shoulders, elbows, wrists, carpophalangeal and interphalangeal joints, hips, knees, tibiotarsic, tarsometatarsic and metatarsophalangeal joints and there were no signs of secondary bone involvement. Considering these findings, we requested an erythrocyte sedimentation rate (ESR) and auto-immunity panel with the following changes: ESR 65mm/h, rheumatoid factor 3160UI/mL and anticitrullinated antibodies 2777UQ. The patient’s clinical and laboratory findings were consistent with a definite diagnosis of RA according to the 2010 ACR/EULAR criteria6 (Table 2) with high activity using the Disease Activity Score-28 with ESR.7 Figure 3 depicts the patient’s hands and feet. Hands X-ray (Fig. 4) showed diffuse osteopenia, boutonniere deformity, erosions on the articular surface of the first metacarpophalangeal joint of the first fingers and erosions with surface destruction of all proximal interphalangeal joints bilaterally. The lung CT findings were also supportive of this diagnosis.8
With an initial fluid restriction strategy and 40 mg of intravenous furosemide daily in an attempt to improve the patient’s urinary output, the sodium levels worsened to 112mEq/L on the 8th hospital day. Afterwards we opted by keeping the fluid restriction combined with 1500mL of isotonic saline with a 4g supplement of hypertonic saline and 40 mg of oral furosemide per day, with some improvement of the sodium levels (120mEq/L on the 12th day). After diagnosing RA, on the 14th day we introduced deflazacort 15 mg/day and methotrexate 15 mg/week, after which we observed a rise in sodium levels up to 132mEq/L on the 18th day. Her hospital stay was complicated by a nosocomial pneumonia and she died on the 23rd day.
Discussion
In this paper we report a case of hyponatremia in the setting of SIADH which, after an extensive workup, proved to be associated with a newly diagnosed RA.
Chronic hyponatremia can present in various non-specific ways, including general malaise, dizziness, confusion or other neurological deficits.9 Our patient had complaints of malaise, fatigue and disorientation to time, which can be partly explained by her hyponatremia, although she also had incipient dementia and an inflammatory underlying condition that could explain some of these symptoms.
Our first challenge in this case was making an appropriate diagnosis of SIADH as the cause of hyponatremia, since it was not easy to determine the volemic state of the patient at first, which implied close observation to ascertain that she was in fact euvolemic. Secondly, the workup for possible causes of SIADH was quite demanding and included many diagnostic tests, since this syndrome has a myriad of causes which either stimulate the release of vasopressin or potentiate its action.2 During an extensive workup, the most frequent causes of SIADH were excluded.
To the best of our knowledge, few reports in the literature mention the association of autoimmune conditions and SIADH and their possible pathogenic mechanisms are also not fully established. Some SIADH reports occurring in the setting of Systemic Lupus Erythematosus have postulated a role of antiphospholipid antibodies in the dysregulation of hypothalamic supraoptic and paraventricular neurons through a non-thrombotic mechanism, with an association with a high disease activity and improvement after immunosuppression.10 Other cases have described a similar association in patients with RA, such as the case of an 84-year-old woman with a recent-onset RA and SIADH whose hyponatremia only improved upon control of the inflammatory arthritis.3 There was also a report of a patient with SIADH and a chronic destructive arthropathy with a persistent rise in CRP and increased IL-6, postulating a role of IL-6 in the excessive release of vasopressin.11 This is supported by a case-report on a child with juvenile idiopathic arthritis and SIADH, in whom hyponatremia only resolved once she was treated with tocilizumab.12 Intravenous IL-6 administration has been shown to stimulate the human hypothalamic-pituitary-adrenal axis, including the release of vasopressin.13 Current data suggests that inflammatory cytokines such as IL-6 and IL-1β have an important influence in the non-osmotic release of vasopressin which, under inflammatory conditions, may be responsible for the development of hyponatremia.14 Based on this, we can speculate that RA created an inflammatory state in our patient with the elevation of cytokines such as IL-6, which in turn stimulated the release of vasopressin leading to SIADH.
Finally, the treatment for SIADH in this patient was quite challenging, with a very modest and slow improvement in sodium levels despite different treatment strategies. These may include fluid restriction, isotonic saline alone or in combination with fluid restriction and, less frequently, hypertonic saline, salt tablets, loop diuretics, vaptans, demeclocycline or urea. Even the most commonly used strategies – monotherapy with either fluid restriction or isotonic saline – have been reported to fail in increasing the serum sodium by 5mEq/L in 55% and 64% of cases respectively, with most patients being discharged from the hospital still hyponatremic.15 Previous studies on inflammatory conditions associated with SIADH have reported that natremia improvement was only possible after treating the underlying condition, with an inverse correlation of sodium levels and inflammatory markers.3,12,16,17 Unfortunately, this follow-up was not possible in our study, since the patient died shortly after. It is therefore hard to ascertain whether RA was the cause of SIADH; however, the growing evidence on the role of IL-6 in both diseases supports this possibility.
To conclude, it is important to raise awareness to the possible association between SIADH and RA. Currently, the paucity of similar reports makes the investigation of other well-established associations to SIADH a priority in its workup. However, if no obvious cause is found and under clinical suspicion, RA should be considered and treated accordingly, since the control of the underlying inflammatory disease might be the only way to successfully treat this syndrome.
Quadro I
Blood workup.
| Variable | Reference range, adults | On admission | 1st hospital day | 4th hospital day | 5th hospital day | 12th hospital day |
| Haematocrit (%) | 35 - 46 | 28 | 27 | 30 | - | 30 |
| Haemoglobin (g/dL) | 12.0 - 15.0 | 9.8 | 9.2 | 9.9 | - | 10.5 |
| Mean globular volume (fL) | 78 - 96 | 77 | 78 | 79 | - | 78 |
| Leucocytes (x10^9/L) | 4.5 - 11.0 | 9.0 | 8.5 | 8.7 | - | 9.6 |
| Platelets (x10^9/L) | 150 - 450 | 184 | 151 | 133 | - | 155 |
| International normalized ratio | 0.80 - 1.20 | 1.36 | - | - | - | - |
| Activated partial-thromboplastin time (seconds) | 25.1 - 36.5 | 31.5 | - | - | - | - |
| Fasting glucose (mg/dL) | 60 - 100 | 166 | - | - | - | - |
| Glycated haemoglobin (%) | <6.5 | - | 5.9 | - | - | - |
| Urea (mg/dL) | 21 - 43 | 17 | 23 | 32 | - | 26 |
| Creatinine (mg/dL) | 0.57 - 1.11 | 0.63 | 0.68 | 0.60 | - | 0.61 |
| Estimated glomerular filtration rate (mL/min/1.73) | >90 | 83 | 80 | 84 | - | 83 |
| Sodium (mEq/L) | 136 - 145 | 124 | 123 | 122 | - | 122 |
| Potassium (mEq/L) | 3.5 - 5.1 | 4.9 | 4.7 | 5.4 | - | 4.3 |
| Chloride (mEq/L) | 98 - 107 | 93 | 90 | 88 | - | 90 |
| Calcium (mg/dL) | 8.4 - 10.2 | 8.4 | 7.9 | 8.2 | - | 8.2 |
| Phosphorus (mg/dL) | 2.3 - 4.7 | 3.0 | 3.2 | 3.8 | - | 2.9 |
| Magnesium (mg/dL) | 1.6 - 2.6 | 1.9 | 1.9 | 1.9 | - | 1.8 |
| C-reactive protein (mg/L) | <5 | 68 | 142 | 78 | - | 78 |
| Brain natriuretic peptide (pg/mL) | <100 | 293 | - | - | - | - |
| Uric acid (mg/dL) | 2.6 - 6.0 | - | 3.4 | - | - | - |
| Total proteins (g/L) | 60.0 - 83.0 | - | 62.5 | - | - | - |
| Albumin (g/L) | 32.0 - 46.0 | - | 25.4 | - | - | - |
| Triglycerides (mg/dL) | <150 | - | 44 | - | - | - |
| Serum iron (μg/dL) | 50 - 170 | - | 10 | - | - | - |
| Transferrin (g/L) | 1.73 - 3.60 | - | 1.23 | - | - | - |
| Saturated transferrin (%) | 20.0 - 40.0 | - | 6.5 | - | - | - |
| Ferritin (ng/mL) | 4.6 - 204.0 | - | 306.5 | - | - | - |
| Thyroid-stimulating hormone (μUI/mL) | 0.35 - 4.94 | - | 3.64 | - | - | - |
| Free thyroxine, FT4 (ng/dL) | 0.70 - 1.46 | - | 0.83 | - | - | - |
| Serum cortisol (μg/dL) | 3.7 - 19.4 (morning) / 2.9 - 17.3 (afternoon) | - | 13.9 | - | - | - |
| Anti-thyroglobulin antibodies (UI/mL) | <4.11 | - | - | 2.53 | - | - |
| Beta-2 microglobulin (mg/L) | 0.97 - 2.64 | - | - | 6.89 | - | - |
| Parathyroid hormone (pg/mL) | 14.8 - 83.1 | - | - | - | 36.1 | - |
| Adrenocorticotropic hormone (pg/mL) | NM - 46 | - | - | - | 53 | - |
| Aldosterone (ng/dL) | 4 - 31 (upright) / 1 - 16 (decubitus) | - | - | - | 10 | - |
| Plasma renin activity (μUI/mL) | 4.4 - 46.1 (upright) / 2.8 - 39.9 (decubitus) | - | - | - | 11.1 | - |
| Erythrocyte sedimentation rate (mm/h) | <16 | - | - | - | - | 65 |
| Folic acid (ng/mL) | 3.1 - 20.0 | - | - | - | - | 4.2 |
| Cobalamin (pg/mL) | 187 - 883 | - | - | - | - | 659 |
| Rheumatoid factor (UI/mL) | <15 | - | - | - | - | 3160 |
| Anticitrullinated antibodies (UQ) | <20 | - | - | - | - | 2777 |
| Anti-neutrophil cytoplasmic antibodies PR3 and MPO (UQ) | <20 | - | - | - | - | <2.3 <3.2 |
| Anti-nuclear antibodies | - | - | - | - | - | Positive 1:160 |
| Extractable nuclear antigen antibodies panel | - | - | - | - | - | Negative |
Quadro II
The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for Rheumatoid Arthritis.
| Criteria | Score | Patient’s score |
| Joint involvement | ||
| 1 large joint | 0 | |
| 2-10 large joints | 1 | |
| 1-3 small joints (with/without involvement of large joints) | 2 | |
| 4-10 small joints (with/without involvement of large joints) | 3 | |
| >10 joints (at least 1 small joint) | 5 | 5 |
| Serology | ||
| Negative RF and negative ACA | 0 | |
| Low-positive RF and low-positive ACA | 2 | |
| High-positive RF or high-positive ACA | 3 | 3 |
| Acute-phase reactants | ||
| Normal CRP and normal ESR | 0 | |
| Abnormal CRP or abnormal ESR | 1 | 1 |
| Duration of symptoms | ||
| Less than 6 weeks | 0 | |
| More than 6 weeks | 1 | 1 |
| Definite diagnosis of Rheumatoid Arthritis | ≥6 | TOTAL SCORE = 10 |
RF- Rheumatoid Factor; ACA- Anticitrullinated Antibodies; CRP- C-Reactive Protein; ESR- Erythrocyte Sedimentation Rate.
Figura I
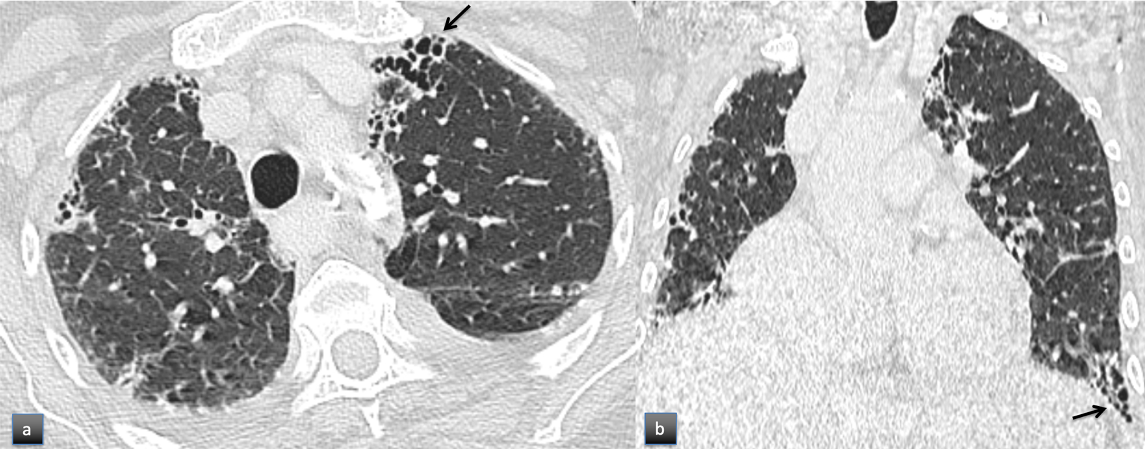
Chest computed tomography in the axial (a) and coronal (b) planes showing mild reticulation, traction bronchiolectasis and honeycombing (arrows), compatible with fibrosis with Usual Interstitial Pneumonia pattern. Discrete bilateral pleural effusion was also present.
Figura II
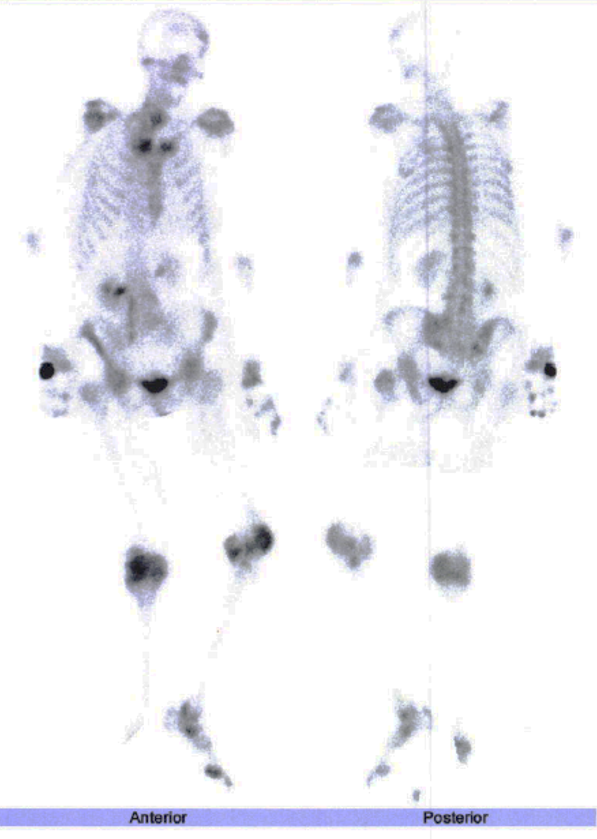
Whole-body bone scintigraphy of the patient.
Figura III
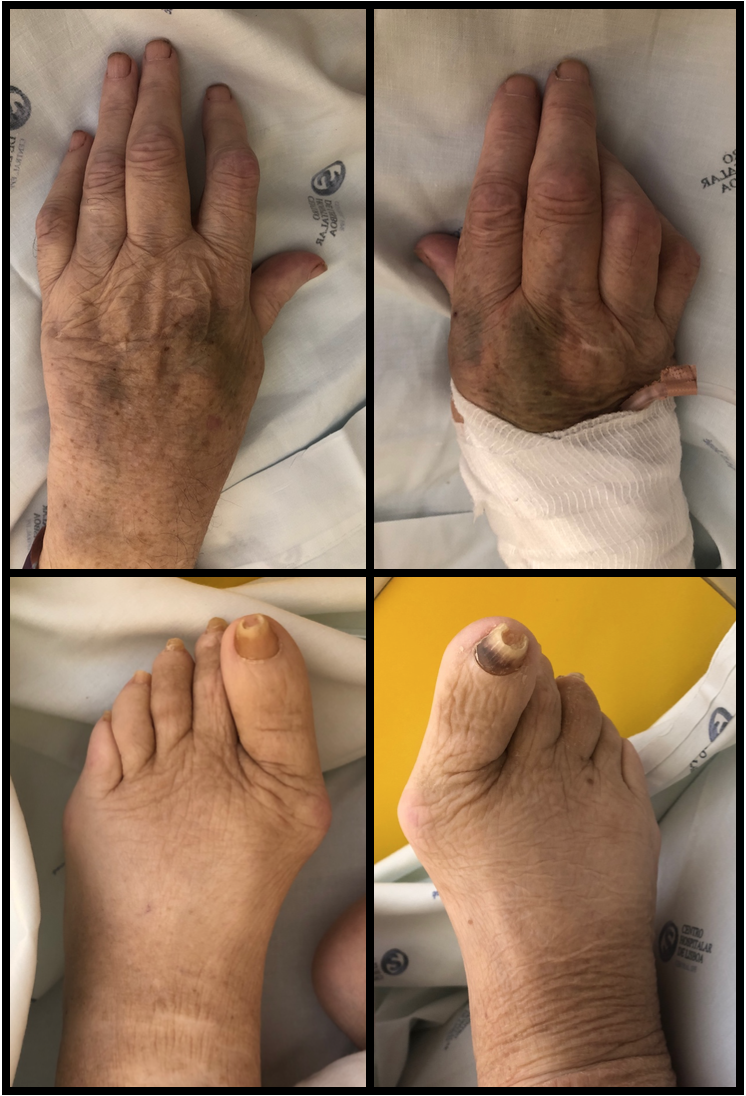
Hands and feet of the patient documented by photography.
Figura IV
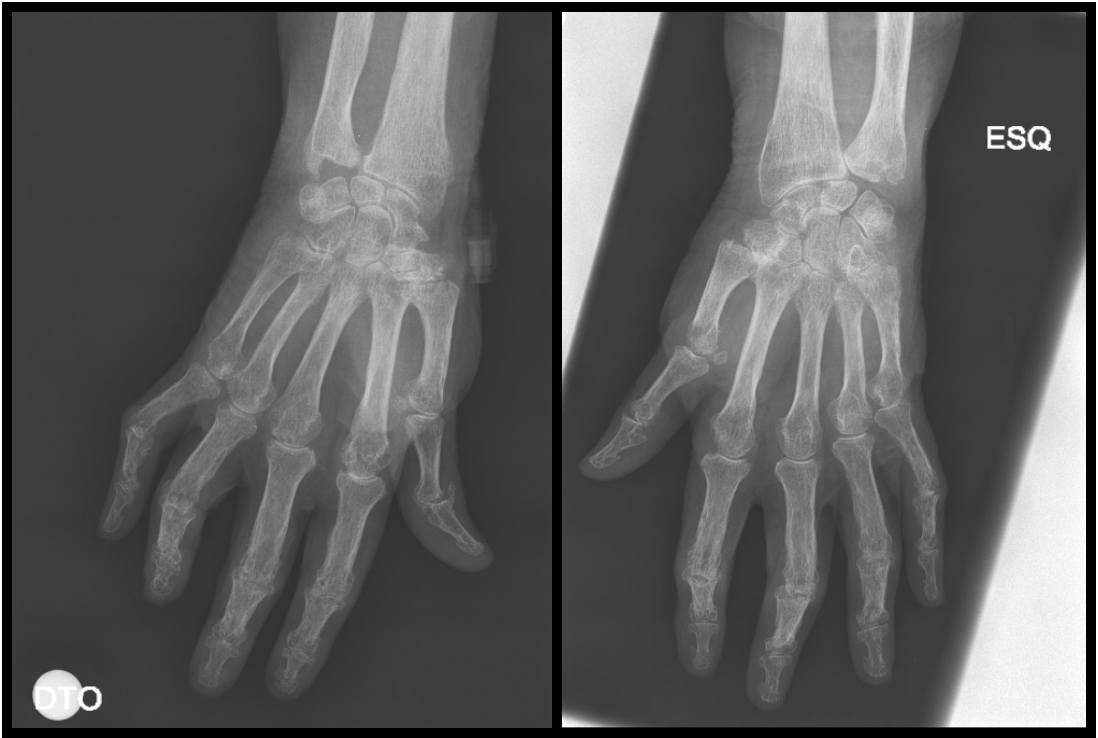
Hands of the patient documented by X-Ray.
BIBLIOGRAFIA
1. Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. 2003;337:169–72.
2. Ellison DH, Berl T. The Syndrome of Inappropriate Antidiuresis. N Engl J Med. 2007;356(20):2064–72.
3. Kaushik V V, Binymin K. Is syndrome of inappropriate antidiuretic hormone secretion an extra-articular manifestation of rheumatoid arthritis? Rheumatology. 2004;43(12):4–5.
4. Narazaki M, Tanaka T, Kishimoto T. The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert Rev Clin Immunol. 2017;00(00):1–17.
5. Swart RM, Hoorn EJ, Betjes MG, Zietse R. Hyponatremia and Inflammation: The Emerging Role of Interleukin-6 in Osmoregulation. Nephron Physiol. 2011;118:45–51.
6. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid Arthritis Classification Criteria. Arthritis Rheum. 2010;62(9):2569–81.
7. Prevoo MLL, Hof MA, Kuper HH, Leeuwen MA, Putte LBA, Riel PLCM. Modified disease activity scores that include twenty‐eight‐joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8.
8. Chansakul T, Dellaripa P, Doyle T, Madan R. Intra-thoracic rheumatoid arthritis: Imaging spectrum of typical findings and treatment related complications. Eur J Radiol. 2015;84(10):1981–91.
9. Peri A, Crohé C, Berardi R, Runkle I. SIADH : differential diagnosis and clinical management. Endocrine. 2017;55(1):311–9.
10. Fabrice GK, Guy D. Inappropriate secretion of ADH and central diabetes insipidus are related to antiphospholipid antibodies in SLE - Case report and review of the literature. Nephrol Dial Transplant. 2006;21(11):3311–5.
11. Murakami T, Matoba H, Kuga Y, Ozawa S, Kubota K, Yoshida S. Hyponatremia in a Patient with Chronic Inflammatory Disease. Intern Med. 1998;37(9).
12. Hodax JK, Bialo SR, Yalcindag A. SIADH in Systemic JIA Resolving After Treatment With an IL-6 Inhibitor. Pediatrics. 2018;141(1):6–11.
13. Mastorakos G, Weber JS, Magiakou M-A, Gunn H, Chrousos GP. Hypothalamic-Pituitary-Adrenal Axis Activation and Stimulation of Systemic Vasopressin Secretion by Recombinant Interleukin-6 in Humans: Potential Implications for the Syndrome of Inappropriate Vasopressin Secretion. J Clin Endocrinol Metab. 1994;79(4):0–5.
14. Park SJ, Shin J Il. Inflammation and hyponatremia: an underrecognized condition? Korean K Pediatr. 2013;56(12):519–22.
15. Verbalis JG, Greenberg A, Burst V, Haymann J. Diagnosing and Treating the Syndrome of Inappropriate Antidiuretic Hormone Secretion. Am J Med. 2016;129(5):537.e9-537.e23.
16. Lim G, Lee M, Kim HS, Hong YM, Sohn S. Hyponatremia and Syndrome of Inappropriate Antidiuretic Hormone Secretion in Kawasaki Disease. Korean Circ J. 2010;40(10):507–13.
17. Fukuda R, Oki M, Itoh M, Komatsu M, Oka A, Ueda A, et al. Syndrome of Inappropriate Antidiuretic Hormone Secretion in Patients with Adult Still’s Disease. Tokai J Exp Clin Med. 2010;35(1):21–4.

