INTRODUCTION
An inflammatory disorder characterized by fever, rash and arthritis was first described in children by George Still in 18961. Almost a century later, a similar entity occurring in adults was published by Bywaters, today known as Adult-onset Still’s disease (AOSD).2It is an uncommon disorder and little information is available about its true incidence.3There are no pathognomonic signs or laboratory findings and, for that reason, several attempts were made to establish diagnostic criteria which included prevalent features like its characteristic evanescent rash, mild oligoarticular arthritis which may evolve to severe destructive polyarthritis, elevated serum ferritin and reduced levels of its glycosylated isoform. However, it is an exclusion diagnosis that requires ruling out infection, malignancy or rheumatologic/auto-immune disorders.4,5The etiology of AOSD is yet unknown as none of the proposed infectious triggers and genetic factors held up.
In celiac disease, arthritis is one of the less frequent extra-intestinal manifestations being present in 2-9% of all cases.6However, cases were described where celiac disease is associated with many auto-immune condition, such as juvenile idiopathic arthritis, rheumatoid arthritis and systemic lupus erythematosus.7,8 To the best of our knowledge, there is only one other case reported in literature where celiac disease associates with AOSD.
CLINICAL CASE:
A 44 year-old Brazilian woman, with no known past medical history, presented to an Emergency Department (ED) complaining of malaise, sore throat, myalgia and high-grade fever for a week. She was dismissed with the diagnosis of urinary tract infection, treated with Trimethoprim-Sulfamethoxazole and later, due to persistent symptoms, amoxicillin/clavulanate. She was admitted in our ED as symptoms progressed, complaining of arthralgia and a fever-related evanescent non-pruritic maculopapular salmon-colored rash (Fig. 1), which started on her face, extending to the arms and legs, sparing palms and soles. The patient lived in a rural environment, having only sporadic contact with cattle. No other epidemiologically relevant data was identified. She mentioned dental manipulation two weeks previously to the beginning of the symptoms.
Physical examination revealed fever (39.3 ºC), an evanescent, non-petechial and non-pruritic, salmon-colored exanthema on her right arm. Left hand small joints, bilaterally and right knee joint were painful, warm and erythematous. No cardiovascular, pulmonary, neurological or other alterations were noticed.
Laboratory findings revealed microcytic hypochromic anemia with hemoglobin level of 10.3 g/dL (reference value [RV] 12.0 – 15.3 g/dL), leukocytosis (13.6x109/L, RV 4.0 – 11.0x109/L) with neutrophil predominance (90.4%), elevated C - reactive protein (CRP) (32.4 mg/dL, RV <0.5 mg/dL) and erythrocyte sedimentation rate was 108 mm/1st h (RV < 12 mm/1st h). Albumin levels were decreased (3.1 g/dL, RV 3.5 – 5.2 g/dL), with normal liver enzymes. Chest roentgenogram and urinalysis were unremarkable.
She was admitted to the Infectious Diseases Department as zoonosis was suspected. She was started on doxycycline, with noticeable oscillating CRP levels and maintenance of low to moderate-grade fever, as further investigations proceeded. Transthoracic echocardiogram revealed a filamentous structure hinting at aortic valve vegetation and empiric treatment for infective endocarditis was started with no benefit to the patient status. Later, transoesophageal echocardiogram revealed Lambl excrescence, discarding the previous diagnosis. Hemocultures and urine cultures were negative. Serology for Brucella, Rickettsia, Lyme disease, Leptospira, Syphilis and antistreptolysin O titers were also negative. Hepatitis virus and HIV were negative.
As she developed dyspnea, cough and lower oxygen saturations needing oxygen therapy, a chest CT was ordered which revealed bilateral axillary and right pulmonary hilum lymphadenopathies, pericardial effusion, multifocal bilateral pulmonary ground-glass opacities predominantly in the upper lobes and bilateral pleural effusion. Abdomen CT showed hepatosplenomegaly and moderate quantity of peritoneal fluid. Thoracocentesis revealed a cloudy sterile transudate with predominance of macrophages. No malignant cells were found neither on pleural fluid nor on bronco-alveolar lavage fluid. Lung and pleural biopsies harvested through flexible bronchofibroscopy and percutaneously, respectively, were normal as well.
On anemia studying we found iron metabolism compatible with iron deficiency, while ferritin levels were high (687 ng/mL, RV 13 – 150 ng/mL), suggesting chronic inflammatory component to the anemia. Folic acid levels were also low. Glycosylated ferritin levels were 28%. Bone marrow biopsy revealed no morphological or maturative abnormalities and PET scan showed no abnormal hypermetabolic foci. Auto-immune markers were also negative. Finding no evidence of infection, malignancy or another auto-immune disorder, we considered the diagnosis of adult-onset Still disease. Symptoms subsided when she was started on indomethacin, the body temperature profile normalized; she was dismissed, and started being followed in external consultation.
Due to gastric complaints, she underwent endoscopy which showed antral gastropathy, bulbitis and duodenitis. Duodenal biopsies revealed villous atrophy, crypt hyperplasia and intra-epithelial T lymphocytes which suggested celiac disease. Anti-transglutaminase (IgA), anti-gliadin (IgA and IgG) were positive. She was started on gluten-free diet and stopped indomethacin after six months. Due to remarkably favorable response to non-steroidal anti-inflammatory drugs, usual progression to corticosteroids and corticoid-sparing immunosuppressant for moderate to severe disease was avoided. After more than 2 years of follow-up, she remains symptom free until today.
DISCUSSION
Although AOSD is an uncommon disease, features like fever, evanescent rash, arthralgia, hepatosplenomegaly and polyserositis are well described in the literature.10,11We have presented this case, not because of atypical manifestations or its well-recognized diagnostic difficulty when such confounding features as Lambl excrescence (causing confusion with infective endocarditis) point to other diagnosis, but for its association with the later found celiac disease. In AOSD, extremely high ferritin levels are usually seen and, in the recent years, it has been recognized as an important feature for diagnosis, especially its glycosylated form, as it is seen in the proposed diagnostic criteria from Fautrel.5In cases like ours, when such low levels of ferritin are present, clinicians seldom think of AOSD.
As hinted by the patient’s low iron and folate levels, the suspicion for some kind of malabsorption syndrome was confirmed by duodenal biopsy, revealing aspects compatible with celiac disease. This suggested a possible mechanism for an only slightly elevated ferritin level in the presence of AOSD, from the lack of availability of iron for ferritin to be secreted into the patient’s circulation, despite the inflammatory stimulus present. Low glycosylated ferritin levels, when compared to total ferritin, made the diagnosis of AOSD more likely, since celiac disease alone seldom occurs with extra-intestinal manifestations.
In inflammatory states, total body iron storage should be increased, but ineffective due to hepcidin overexpression, hindering iron’s utilization.12We theorize that the presence of true iron deficiency stunted the inflammatory raise of serum ferritin.
The available literature focuses mainly on extremely high levels of ferritin as a diagnostic tool, in such magnitude that are only usually seen in hemophagocytic syndrome and AOSD.13As our case suggests, AOSD doesn’t require a cutoff level of ferritin, underlining the significance of utilizing glycosylated ferritin as a surrogate and more accurate diagnostic tool. Although in inflammatory states ferritin levels usually rise, the percentage of glycosylated ferritin doesn’t seem to proportionally elevate, remaining at low levels. Some authors have observed that glycosylated ferritin levels are significantly decreased in AOSD in comparison to other inflammatory disorders, although its mechanism is not yet fully understood.13
In disease absent states, 50-80% of ferritin is in glycosylated form. When inflammation is present, the glycosylated proportion decreases, possibly due to saturation or failure of the glycosylation mechanism. Nevertheless, such decrease is never as abrupt as in AOSD.13,14When the association between a high serum ferritin level and a very low glycosylated ferritin level is present, the diagnosis of AOSD is much more likely.13–15
If the suspicion for an auto-inflammatory condition is high, slight to moderate increases in serum ferritin, should not exclude AOSD diagnosis. Decreased glycosylated ferritin levels may aid the diagnosis, especially in such cases where serum ferritin elevation is stunted due to any malabsorption or inability to increase its production in inflammatory states. In such cases, true iron deficiency should raise suspicion of a super-imposed condition other than the primary inflammatory disease.
Due to the low incidence in the literature about the association between celiac disease and AOSD (only one other case),
9we can only conclude a fortuitous relationship is in the background for the events described. We underline the importance of reporting cases when low iron storages stunt the ability to produce ferritin, to better understand the mechanism involved and the diagnosis of AOSD.
Figura I
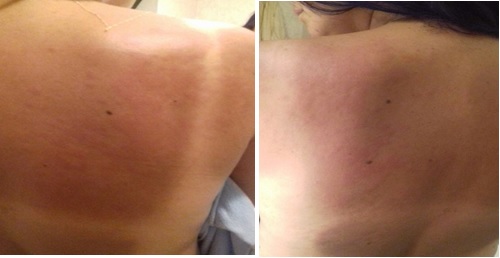
Evanescent rash on the patient’s back.
BIBLIOGRAFIA
1. Still George F. Form of Chronic Joint Disease. 1896.
2. Bywaters EGL. Still ’ s disease in the adult. Ann Rheum Dis. 1971;30:121-33.
3. Evensen KJ, Nossent HC. Epidemiology and outcome of adult-onset Still’s disease in Northern Norway. Scand J Rheumatol. 2006;35(1):48-51. doi:10.1080/03009740510026616
4. Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424:2014.
5. Faurel B, Zing E, Golmard J, Le Moel G, Bissery A, Rioux C, et al. Proposal for a New Set of Classification Criteria for Adult-Onset Still Disease. J Rheumatol.1992 Mar;19(3):424-30.
6. Laurikka P, Nurminen S, Kivelä L, Kurppa K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients. 2018;10(8):1015. doi:10.3390/nu10081015
7. Stagi S, Giani T, Simonini G, Falcini F. Thyroid function, autoimmune thyroiditis and coeliac disease in juvenile idiopathic arthritis. Rheumatology. 2005;44(4):517-20. doi:10.1093/rheumatology/keh531
8. Warjri SB, Ete T, Beyong T, Barman B, Lynrah KG, Nobin H, Perme Obang. Coeliac Disease With Rheumatoid Arthritis: An Unusual Association. Gastroenterol Res. 2015;8(1):167-8. doi:10.14740/gr641w
9. Kumar S, Gupta N, Jhamb R, Mishra D. Celiac disease: Association with adult-onset Still’s disease: Apropos of a clinical case. Indian J Med Sci. 2007;61(7):414. doi:10.4103/0019-5359.33191
10. Fong WS, Lui NL. Adult-onset still’s disease: A review. Proc Singapore Healthc. 2013;22(1):40-7. doi:10.1177/201010581302200107
11. Efthimiou P, Paik PK, Bielory L. Diagnosis and management of adult onset Still’s disease. Ann Rheum Dis. 2006;65(5):564-72. doi:10.1136/ard.2005.042143
12. Wang W, Knovich MA, Coffman LG, Torti FM, Torti S V. Serum Ferritin: Past, Present and Future. Biochim Biophys Acta. 2011;1800(8):760-9. doi:10.1016/j.bbagen.2010.03.011.Serum
13. Fautrel B, Moël GLE, Saint-marcoux B, Taupin P, Vignes S, Rozenberg S, et al. Diagnostic Value of Ferritin and Glycosylated Ferritin in Adult Onset Still ’ s Disease. J Rheumatol. 2001;28(2).
14. Mehta B, Efthimiou P. Ferritin in Adult-Onset Still ’ s Disease : Just a Useful Innocent Bystander ? International Journal of Inflammation. 2012;2012. doi:10.1155/2012/298405
15. Vignes S, Moël G Le, Fautrel B, Wechsler B, Godeau P, Piette J. Percentage of glycosylated serum ferritin remains low throughout the course of adult onset Still ’ s disease. Ann Rheum Dis. 2000; 59:347-50.


