INTRODUTION
Acute Eosinophilic Pneumonia (AEP) is a rare disease, with an acute onset (fever, cough and dyspnea) and hypoxemia, due to a pulmonary eosinophilic infiltrate triggered by exposure to an inhaled antigen, medication or infection 1,2,16. This is an important entity to take into account in the differential diagnosis of acute respiratory failure1. The early initiation of corticosteroid therapy in patients with moderate to severe disease allows for an optimal prognosis, with rapidly improve in days, on the contrary if not treated could be fatal 3,16,18.
CASE REPORT
A 76-year-old woman, without previous pulmonary diseases or smoking habits, presented to the emergency department with 1-week history of non-productive cough, dyspnea in exercise, malaise and fever (over 38ºC). The symptoms started after a big home cleaning with exposure to dust. She denies start of new drugs and contact with birds, silica or travels. The patient was discharged medicated with doxycycline 100 mg/per day, orally for 10 days. Despite this, after 10 days, she presented clinical worsening, with dyspnea at rest and persistent fever. She returned to the emergency department. On examination, she presented fever (38.8ºC), tachypnea, saturation on room air of 80%. Pulmonary auscultation with bibasal inspiratory crackles and a decrease auscultation on the bases. Remaining clinical observation was unremarkable.
Chest radiography (Figure 1a) on admission showed areas of heterogeneous consolidation with air bronchogram, of ill-defined limits, with predominantly peripheral distribution, in the middle and lower zone of both lung fields.
On laboratory evaluation, she had leukocytosis (15,7x103/µL), neutrophilia (10,8x103/µL) and eosinophilia (1,82x103/µL). C-reactive protein was raised (147mg/dL) and procalcitonin was normal.
The patient was hospitalized with the hypothesis of acquired community pneumonia and medicated with piperacillin-tazobactam 4.5mg every 6h intravenous and oxygen therapy.
Despite the antibiotherapy, the patient presented a worsening of respiratory failure and persistence of fever (Figure 3). Chest radiography had a worsening of infiltrates (Figure 1b) and a chest computed tomography (CT) (Figure 2a) was requested, which showed an extensive bilateral consolidation with air bronchogram (alveolar filling) and bilateral ground-glass opacities and thickening of peribroncovascular soft tissue. The lesions distribution is predominantly peripheral, in the middle and lower zone of both lung fields. She presented bilateral pleural effusions.
The patient was admitted to the intensive care unit (ICU) and initiated therapy with High-Flow Nasal Oxygen (HFNO). Intravenous fluconazole 400mg/day and clarithromycin 500mg twice a day were added.
A bronchoscopy was performed with bronchoalveolar lavage (BAL). BAL fluid presented eosinophilia (41%) and lymphocytosis (42%), with CD4 predominance. BAL polymerase chain reaction (PCR) did not detect Mycobacterium tuberculosis. The culture of BAL was negative to bacteria and fungus.
Blood cultures did not show growth. Urinary antigens for Streptococcus pneumoniae and Legionella pneumophila were negative. Serologies to Mycoplasma pneumoniae, Varicella-Zoster, Chlamydia pneumoniae, Toxoplasma gondii, Epstein-Barr, Parvovirus B19 and Herpes Simplex were negative to active infection. Influenza A and B were negative. HIV and hepatitis virus were excluded.
Given the clinical presentation, radiological findings and eosinophilic infiltration in BAL, a final diagnosis of acute eosinophilic pneumonia was made. The patient was medicated with corticosteroid therapy (methylprednisolone 125mg every 6 hours during 3 days followed by Prednisolone 1mg/kg/day).
After 3 days of corticosteroid therapy the patient had a significant improvement, with reduction on oxygen support, and was transfer to pneumology department. Radiologic re-evaluation demonstrated a significant reduction of the opacities (Figure 1c). The patient was discharged on the 10th day of prednisolone. She was medicated with oxygen 2L/minute 24hours/day and prednisolone 40mg/day.
One month later, the patient presented complete resolution of respiratory failure and a significant improvement in the chest radiography (Figure 1d). The corticosteroid tapering was initiated.
Three months after discharge, the patient remained asymptomatic. Computed tomography was performed (Figure 2b) and showed small areas of parenchymal densification with nearly complete resolution of the inflammatory process.
DISCUSSION
This case is a great example of the challenge of differential diagnosis in acute respiratory failure. Despite the high prevalence of bacterial pneumonias, it is important to be highly suspicious for rarer diagnoses especially when there is no improvement after antibiotics.
The AEP is an eosinophilic infiltration of the pulmonary parenchyma, whose cause is unknown. It is assumed that it is a hypersensitivity reaction to an inhaled antigen, drugs or infections, that causes alveolar damage and subsequent inflammatory reaction that culminates in the activation and recruitment of eosinophils1, 2, 4, 18-20.
The inflammatory reaction mediated by eosinophils develops in phases: in the initial phase, the presence of allergens in the airway leads to sensitization of submucosal dendritic cells and the consequent presentation of B cells with IgE release. In the next phase, mast cells are activated by IgE and consequently release inflammatory proteins, resulting in the recruitment of eosinophils16;18.
Coincidentally, our patient developed symptoms 1 week after a major cleaning with exposure to dust and other possible causes were excluded. That association is already recognized 1, 2, 5, 6. However, the most common association with AEP development is with smoking (start or change of frequency)1, 2, 17. It should be noted that in some cases there is no identifiable cause or its association is difficult to prove.1, 2 In these situations, idiopathic eosinophilic pneumonia is assumed. 1, 2
It is not characteristic in AEP patients the presence of allergic diseases in the past16.
The AEP is a rare entity of acute respiratory failure. The diagnosis is based on the modified Philit criteria: (1) less than 1 month in duration; (2) pulmonary infiltrates on the chest radiography or CT; (3) eosinophils more than 25% in BAL fluid or eosinophilic pneumonia on lung biopsy and (4) absence of the other specific pulmonary eosinophilic diseases1.
Patients develop symptoms of acute onset (less than a month, but usually 7 days) with dyspnea, fever and non-productive cough 2, 3, 5–8. The severity of the disease is very heterogeneous, some cases present with severe respiratory failure that requires ICU admission and mechanical ventilation1, 2. The percentage of patients with severe disease varies in different retrospective studies (16-30%) 2, 5, 6.
Our patient was admitted to the ICU due to worsening respiratory failure. Based on current scientific knowledge about the safety and efficacy of HFNO in hypoxemia9–11, HFNO was successful in our patient, avoiding mechanical ventilation.
AEP radiologic features are quite characteristic with presence of bilateral reticular opacities, the Kerley B-Lines at the chest radiography and bilateral patchy areas of ground-glass with or without consolidation opacities and smooth interlobular septal thickening at the CT 1, 2, 5, 7, 8; 16. Bilateral pleural effusion is very common and, when evaluated, pleural fluid has an exudative characteristic and cytology shows an eosinophilic increase 1.
The presence of more than 25% of eosinophils in the BAL practically makes the diagnosis, but an increase in neutrophils and lymphocytes could be present1, 2, 12. In our case, the lymphocytosis was markable, this fact has already been described in previous publications as a review of 15 cases where the average lymphocytosis in the BAL was 20.7%, with cases reaching 34% 13. In other case, EP induced by vancomycin, the BAL presented with 24% of lymphocytes14. BAL lymphocytosis is characteristic of granulomatous diseases, such as sarcoidosis or acute hypersensitivity pneumonitis, but none of these are characterized by the rise in eosinophils, thus making this diagnosis less likely15.
Other characteristic is the peripheral eosinophilia but it may not be present at the diagnosis but develops during the following days1, 7.
Due to the rarity of the disease, there are no studies with large samples, so there is no consensus regarding the therapeutic protocol3. There are some cases described with spontaneous resolution without therapy3, 6. In severe cases, therapy with intravenous corticosteroids (methylprednisolone 60-125mg every 6 hours for 3 days, followed by switching to oral therapy) is recommended. In moderate to mild cases, oral therapy is considered sufficient (prednisolone 1mg/kg/day)1, 3. The duration of treatment was generally 4 weeks (including tapering), but some studies have shown no inferiority to treatment at 2 weeks2, 3.
After the initiation of therapy, the patient presented a rapid clinical improvement. This is coincident with described in other cases, generally with resolution of infiltrates on chest radiography after one month, without sequelae 1, 2, 8.
Figura I
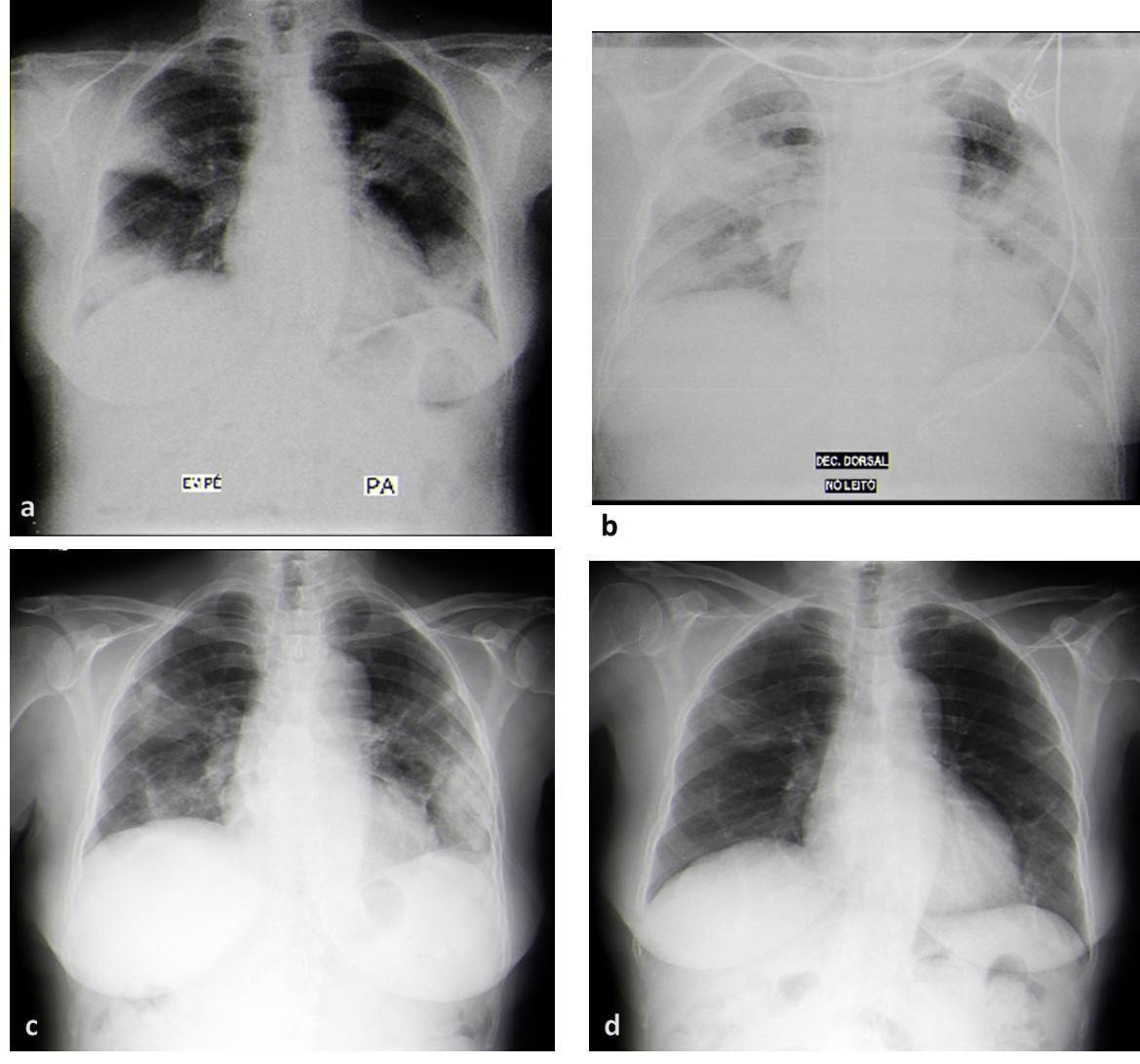
a) Chest radiography (posterior-anterior) in the emergency department with areas of heterogeneous consolidation with air bronchogram, of ill-defined limits, with predominantly peripheral distribution, in the middle and lower zone of both lung fields; b) The chest radiography (anterior-posterior) at 4th day of hospitalization present a worsening of infiltrates; c) Chest radiography (posterior-anterior) after six days under corticotherapy demonstrated a significant reduction in infiltrates, still showing some bilateral reticular infiltrates. d) Chest radiography (posterior-anterior) one month after discharge, with almost complete resolution of the infiltrates.
Figura II
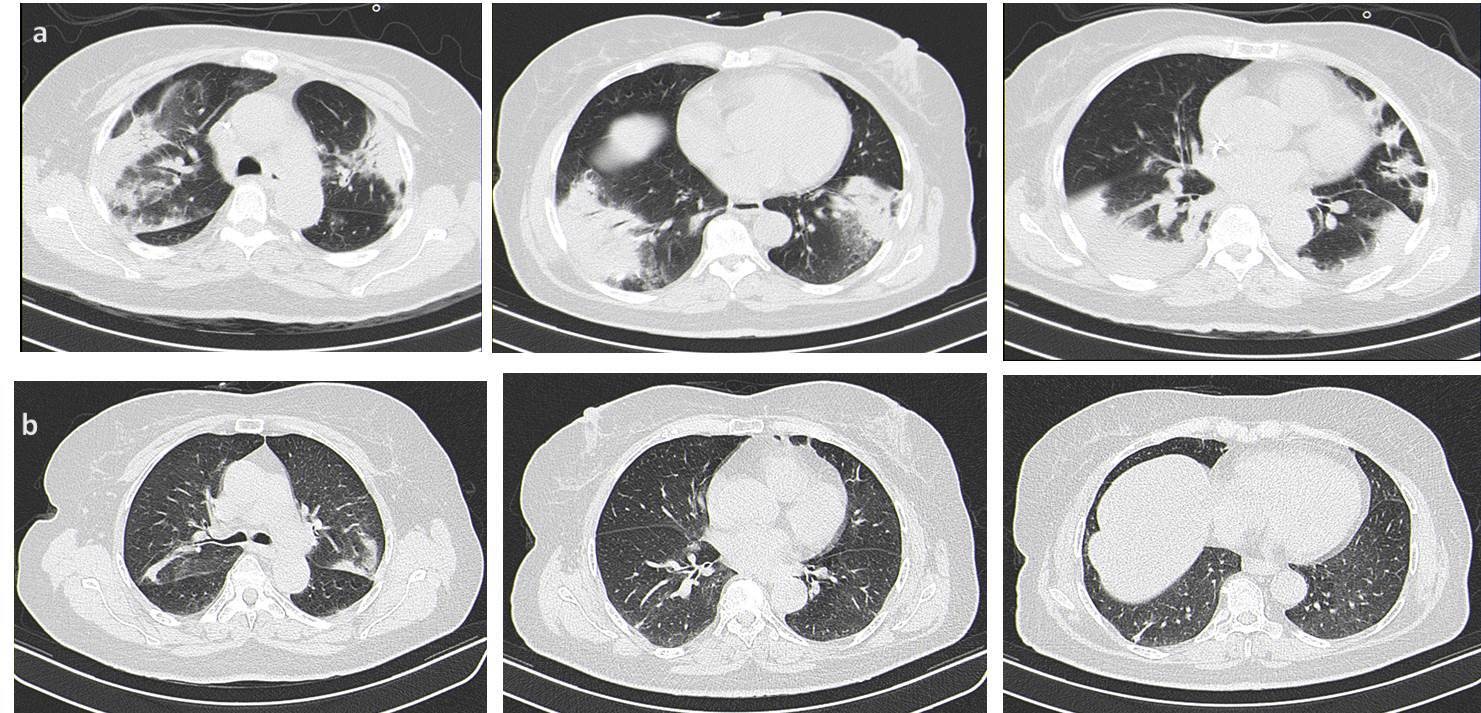
a) Chest computed tomography (CT) showed an extensive bilateral consolidation with air bronchogram (alveolar filling), bilateral ground-glass opacities and thickening of peribroncovascular soft tissue and bilateral pleural effusions. The lesions distribution is predominantly peripheral, in the middle and lower zone of both lung fields. b) High-Resolution Computed tomography, three months discharge, showed small areas of parenchymal densification with nearly complete resolution of the inflammatory process.
Figura III
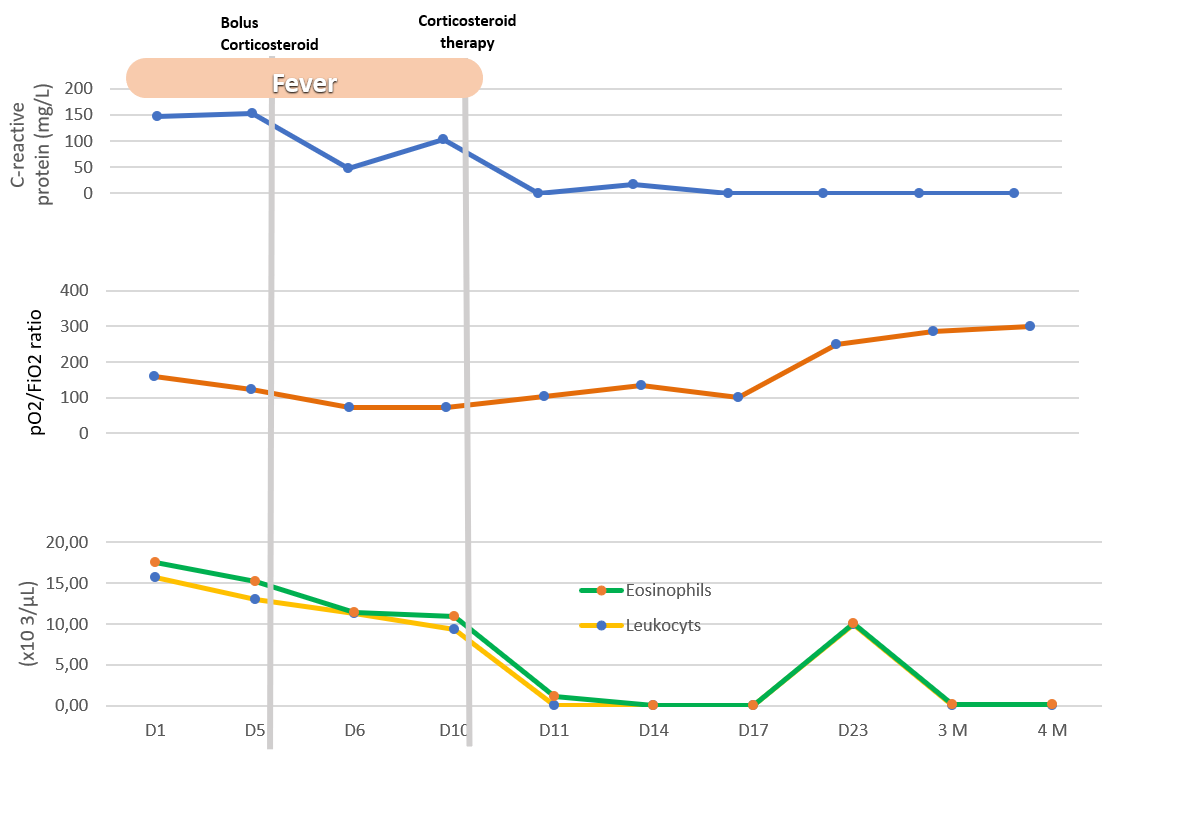
Graph illustrating clinical and analytical evolution. D, Day of hospitalization; M, mouth follow up.
BIBLIOGRAFIA
[1] De Giacomi F, Vassallo R, Yi ES, et al. Acute eosinophilic pneumonia. Am J Respir Crit Care Med 2018; 197: 728–736.
[2] Suzuki Y, Suda T. Eosinophilic pneumonia: A review of the previous literature, causes, diagnosis, and management. Allergol Int 2019; 68: 413–419.
[3] Rhee CK, Min KH, Yim NY, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J 2013; 41: 402–409.
[4] Cottin V. Eosinophilic Lung Diseases. Clin Chest Med 2016; 37: 535–556.
[5] Ajani S, Kennedy CC. Idiopathic acute eosinophilic pneumonia: A retrospective case series and review of the literature. Respir Med Case Reports 2013; 10: 43–47.
[6] Philit F, Etienne-Mastroïanni B, Parrot A, et al. Idiopathic acute eosinophilic pneumonia: A study of 22 patients. Am J Respir Crit Care Med 2002; 166: 1235–1239.
[7] Badesch DB, King TE, Schwarz MI. Acute eosinophilic pneumonia: A hypersensitivity phenomenon? Am Rev Respir Dis 1989; 139: 249–252.
[8] Pizzuto M, Seychell M, Caruana Montaldo B, et al. Idiopathic acute eosinophilic pneumonia. BMJ Case Rep 2019; 12: 1–5.
[9] Zhu Y, Yin H, Zhang R, et al. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients with acute respiratory failure: A systematic review and meta-analysis of randomized controlled trials. BMC Pulm Med 2017; 17: 1–10.
[10] Fealy N, Osborne C, Eastwood GM, et al. Nasal high-flow oxygen therapy in ICU: A before-and-after study. Aust Crit Care 2016; 29: 17–22.
[11] Messika J, Ahmed K Ben, Gaudry S, et al. Use of high-flow nasal cannula oxygen therapy in subjects with ARDS: A 1-year observational study. Respir Care 2015; 60: 162–169.
[12] Sohn JW. Acute eosinophilic pneumonia. Tuberc Respir Dis (Seoul) 2013; 74: 51–55.
[13] Pope-Harman AL, Davis BW, Allen ED, et al. Acute Eosinophilic Pneumonia A Summary of 15 Cases and Review of the Literature. Medicine (Baltimore) 1996; 75: 334–342.
[14] Isono T, Sawaguchi H, Kusumoto H, et al. Eosinophilic pneumonia putatively induced by vancomycin: A case report. Am J Case Rep 2019; 20: 1440–1445.
[15] Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012; 185: 1004–1014.
[16] Nakagome K, Nagata M. Possible Mechanisms of Eosinophil Accumulation in Eosinophilic Pneumonia. Biomolecules. 2020;10(4):638.
[17] Chaaban T. Acute eosinophilic pneumonia associated with non-cigarette smoking products: a systematic review. Advances in Respiratory Medicine. 2020;88(2):142-146.
[18] Allen J, Wert M. Eosinophilic Pneumonias. The Journal of Allergy and Clinical Immunology: In Practice. 2018;6(5):1455-1461.
[19] Felton J, Lucas C, Rossi A, Dransfield I. Eosinophils in the Lung - modulating apoptosis and efferocytosis in airway inflammation. Frontiers in Immunology. 2014;5.
[20] Storandt M, Matta A. Acute Eosinophilic Pneumonia: A Rare Complication of Daptomycin Therapy. Cureus. 2020; 17 (1):e6803.




