Introduction
DRESS Syndrome is a severe, potentially life-threatening hypersensitivity reaction to drugs that presents with rash, hematological changes (leukocytosis with eosinophilia or atypical lymphocytes) and multiorgan involvement (most commonly liver or renal dysfunction). Although evidence is limited, incidence may range from 1/1000 to 1/10.000 drug exposures with a mortality rate of ~10%1. Reported cases commonly affect adults, most often associated with antiepileptics and allopurinol2,3. Delayed onset (2-8 weeks after starting the drug) and longer resolution time is characteristic. The syndrome pathogenesis is still not certain. Current knowledge is that several human leukocyte antigen (HLA) genotypes, slow acetylation metabolic pathways and viral reactivation (Epstein-Barr Virus (EBV), Cytomegalovirus (CMV), human herpesvirus (HHV)-6 and HHV-7) might contribute in a complex interaction for this syndrome to occur4.
Sulfasalazine is a 5-aminosalicylic acid derivate increasingly used in inflammatory diseases. Adverse reactions are common, usually nonserious and dose related, mostly gastrointestinal, headache or skin rash. Sulfonamides, particularly sulfasalazine, have been described as possible causes of DRESS syndrome. Here we present a severe case with visual support of this rare and potentially life-threatening complication.
Clinical case
A 42-year-old woman presented with fever and erythematous rash, initially localized in the trunk, but later extended to the entire body surface. She was being studied for suspected polyarthritis, reason why she had started sulfasalazine about 5 weeks earlier (500 mg/day, during that time increased to 2500mg/day). Initially it was assumed as possible cutaneous manifestation related to the polyarthritis under study, and the patient was treated with prednisolone 1.5mg/kg/day, cetirizine 10mg/day and suspended sulfasalazine. Due to the persistence and exacerbation of the rash, she was admitted to our department 5 days later. The physical examination showed enlargement of the liver and spleen, as well as of cervical, occipital and posterior auricular lymph nodes. Pictures of maculopapular rash are presented in figure 1.
Laboratory tests revealed eosinophilia and lymphocytosis (smear with hyperbasophilic cytoplasm lymphocytes, irregular contours and peripheral basophilia) and hepatic cytocholestasis. Eosinophilia, hepatic cytocholestasis and inflammation markers increased significantly on the following 10 days reaching: eosinophilia 3800/µL; C-reactive protein, 9.94 mg/dL (N<0.5mg/dL), erythrocyte sedimentation rate, 73 mm/h (N< 15); lactate dehydrogenase 588IU/L (N<214 IU/L); aspartate aminotransferase 569IU/L (N<27 IU/L); alanine transaminase 341 IU/L (N<34IU/L); alkaline phosphatase 329 IU/L (N<104 IU/L); gamma-glutamyltransferase 739 IU/L (N<61 IU/L); total bilirubin 6.16 mg/dL (N<1.1 mg/dL). A skin biopsy was also performed and showed a dermal perivascular inflammatory infiltrate (lymphocytes, histiocytes and eosinophils) with negative immunofluorescence for IgA, IgG, IgM, C3 e C1q, supporting the diagnosis of DRESS Syndrome, and excluded cutaneous lymphoma. Serological tests were negative for Toxoplasmosis, Borrelia, Mycoplasma, CMV, HHV6, Parvovirus, Leptospira, Rickettsia, HIV and hepatitis A, B and C. Tests for autoimmune antibodies (antinuclear, anti-neutrophil cytoplasmic antibodies, anti- cyclic citrullinated peptide, anti-soluble liver antigen and rheumatoid factor) were also negative. IgM and IgG for EBV virus were also negative but DNA PCR was positive.
Patient was treated with corticosteroid therapy (Prednisolone 1mg/kg/day IV), antihistamine and was under immunoglobulin for 3 days on the first week of treatment. After small improvements interrupted by exacerbations on the first 10 days, that leaded to temporary transfer to an intermediate care unit, she improved clinically slowly with several relapses, being discharged after 40 days with prednisolone being tapered to over the next 2 months. No recurrence occurred on the next year of follow-up. About 18 months later the patient was under study due to a suspected hypersensitivity to isoniazid.
Discussion
Sulfonamides, mostly sulfasalazine, have been reported as frequently associated with DRESS syndrome. A review, from 2016, found less than 50 cases reported worldwide5. Although the etiology is not clear, certain human leukocyte antigen (HLA) haplotypes might increase susceptibility. Particularly for sulfasalazine, the haplotype HLA-B*13:01 has some evidence of association6.
Our patient was diagnosed with a sulfasalazine induced DRESS syndrome. The multisystemic involvement and eosinophilia, important characteristics of the syndrome, were present. Time of presentation (5 weeks after drug start), fever with suggestive progressive erythematous rash, blood smear with atypical lymphocytes, eosinophilia, lymphadenopathy, inflammatory infiltrate in skin biopsy, hepatic involvement and prolonged course with relapses, even with drug discontinuation, are also all features in favor of the diagnosis.
Viral serology in immunocompromised patients is not always easy to analyze. A negative serology for EBV, even if there was a previous infection, is not rare. A DNA detection using PCR favors the diagnosis of reactivation, although there is lack off standardization in methods to value and report results that could mislead interpretation7. In sulfasalazine induced DRESS syndrome, HHV-6 reactivation is common (81%5), which did not occur. However, EBV reactivation, less likely (<10%), was probably present5,8. Evidence is not clear regarding virus reactivation being part of the possible etiology or, on the other hand, a consequence of T-lymphocytes cross reaction8. Despite this, virus reactivation seems to indicate higher risk for prolonged disease course and complications9.
Syndrome mortality (5-10%3,10) is related to organ involvement, mostly fulminant hepatitis, interstitial nephropathy, eosinophilic interstitial pneumopathy, pericarditis, myocarditis or pancreatitis11. Regarding our patient, liver was the most affected organ with worsening parameters on the first 10 days, which motivated a transient transfer to the intermediate care unit. The treatment is not standardized. Drug withdrawal is the first immediate measure and, to do so, it implies clinical awareness and high suspicion at patient presentation. Although widely used, systemic corticosteroids have not been evaluated in randomized trials and dose range is not standardized. We used prednisolone 1mg/kg/day (as well as immunoglobulin for 3 days) at start, with a histamine antagonist. Tapering down the dose was slowed after symptoms worsening at first reduction after 3 weeks treatment. Overall, our patient took corticosteroids for over 100 days. Intravenous immunoglobulins have limited and conflicting evidence of benefit on single use12. In association, as we used, it might be beneficial13, although more robust evidence is needed. At discharge, hepatic markers parameters were the only ones not normalized, but had recovered fully at the end of the treatment. No recurrence was reported at least on the next year, what would be expected, as it is rare and more common with anticonvulsive. However, interestingly, over one year after discharge, the patient is currently under study due to a suspected hypersensitivity to isoniazid. Although having being reported to be more common on the first months after the diagnosis of DRESS syndrome, there is evidence of an increased risk of reaction to structurally unrelated drugs14, all well as association with autoimmune diseases (diagnosed before and after the DRESS syndrome occurrence15,16). All this data supports the idea that DRESS syndrome pathogenesis relates to immunogenicity alterations beyond cross reaction to a single drug.
Conclusion
The diagnosis of DRESS syndrome implies high level of suspicion. It affects young patients and is associated to prolonged hospitalization and significant mortality risk. Here we report a typical presentation to a not so typical medicine. Drug prompt withdrawal and organ support is essential. Better understanding of the syndrome pathogenesis shall allow us to standardize treatment, as it still remains empirical and with no established regimens.
Data confidentiality
Informed consent was duly obtained from the patient.
Figura I
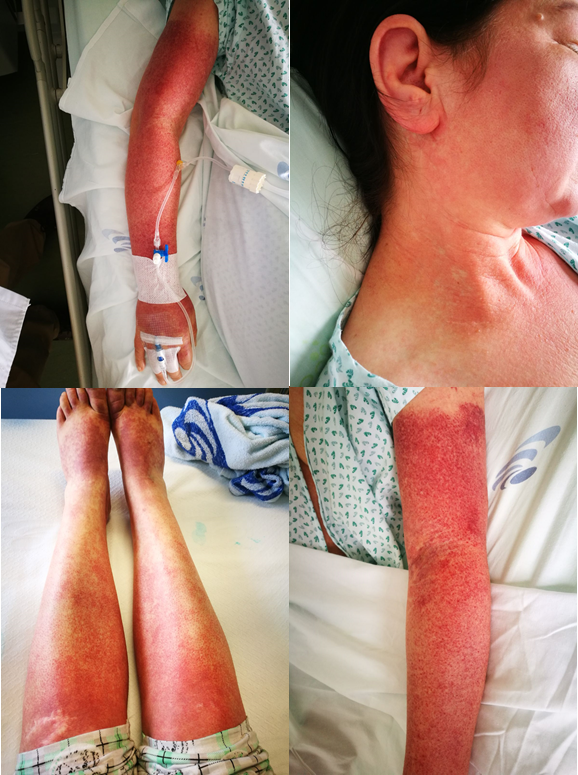
Patient’s cutaneous manifestations at day 5 of symptoms
BIBLIOGRAFIA
References
1. Cacoub P, Descamps V, Meyer O, Speirs C, Belissa-Mathiot P, Musette P. Drug rash with eosinophilia and systemic symptoms (DRESS) in patients receiving strontium ranelate. Osteoporos Int 2013;24:1751-7.
2. Watanabe H. Recent Advances in Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms. J Immunol Res 2018;2018:5163129.
3. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011;124:588-97.
4. Choudhary S, McLeod M, Torchia D, Romanelli P. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome. J Clin Aesthet Dermatol 2013;6:31-7.
5. Krishan P, et al. Sulfasalazine Induced DRESS Syndrome: A Review of Case Reports. Journal of Advances in Medicine and Medical Research 2016.
6. Yang F, Gu B, Zhang L, et al. HLA-B*13:01 is associated with salazosulfapyridine-induced drug rash with eosinophilia and systemic symptoms in Chinese Han population. Pharmacogenomics 2014;15:1461-9.
7. AbuSalah MAH, Gan SH, Al-Hatamleh MAI, Irekeola AA, Shueb RH, Yean Yean C. Recent Advances in Diagnostic Approaches for Epstein-Barr Virus. Pathogens 2020;9.
8. Roujeau JC, Dupin N. Virus Reactivation in Drug Reaction with Eosinophilia and Systemic Symptoms (Dress) Results from a Strong Drug-Specific Immune Response. J Allergy Clin Immunol Pract 2017;5:811-2.
9. Tohyama M, Hashimoto K, Yasukawa M, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol 2007;157:934-40.
10. Chen YC, Chiu HC, Chu CY. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol 2010;146:1373-9.
11. Peyriere H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol 2006;155:422-8.
12. Joly P, Janela B, Tetart F, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol 2012;148:543-4.
13. Giavina-Bianchi P, and Shih-Wen Huang. Intravenous Immunoglobulin May Be Beneficial as an Add-on Therapy in DRESS. The Journal of Allergy and Clinical Immunology: In Practice 2018:1243-5.
14. Picard D, Vellar M, Janela B, Roussel A, Joly P, Musette P. Recurrence of drug-induced reactions in DRESS patients. J Eur Acad Dermatol Venereol 2015;29:801-4.
15. Ushigome Y, Kano Y, Ishida T, Hirahara K, Shiohara T. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol 2013;68:721-8.
16. Chen YC, Chang CY, Cho YT, Chiu HC, Chu CY. Long-term sequelae of drug reaction with eosinophilia and systemic symptoms: a retrospective cohort study from Taiwan. J Am Acad Dermatol 2013;68:459-65.


