INTRODUCTION
Hyponatremia, defined as serum sodium concentration < 135 mmol/L, is a very common disorder and isassociated with increased mortality and morbidity.1Hypopituitarism results from deficiency in pituitary hormones and is caused by diseases that reduce or destroy the secretory function or interfere with the hypothalamic secretion of pituitary-releasing hormones.2It’s a rare cause of hyponatremia, often misdiagnosed, especially in the elderly, since its clinical picture is frequently insidious and non-specific.3
Empty sella is characterized by the herniation of the subarachnoid space within the sella, associated with a degree of flattening of the pituitary gland.4It ismore common in women, with a peak incidence between 30 and 40 years, with endocrine abnormalities documented in only 19% of the patients.4
CASE DESCRIPTION
A 68-year-old man, with a medical history of essential hypertension, in use of irbesartan and hydrochlorothiazide, presented at the emergency room due to malaise, asthenia, somnolence and anorexia for the previous 2 days.
At admission he was vigil but lethargic, his blood pressure was 138/72 mmHg, heart rate 78 beats/min, body temperature of 36.4oC. He was pale, presented with hair weakening, mainly on his eyebrows, from which the external third was missing.
Blood chemistry revealed severe hyponatremia (108 mmol/L), normal potassium levels, a reduced serum osmolarity (220 mosmol/L), a normal renal function, serum glucose, triglycerides and unremarkable serum protein electrophoresis. It also showed a urine osmolarity <100 mosmol/L and a high urinary sodium.
Isotonic fluids were started with poor response. Hence hormonal investigation ensued, showing low levels of adrenocorticotropic hormone (ACTH) and cortisol, high levels of thyroid-stimulating hormone (TSH) and a very low free thyroxine (FT4), low growth hormone (GH), luteinizing hormone (LH) and testosterone and also a borderline low follicle-stimulating hormone (FSH) and insulin-like growth factor-1 (table 1).
Hypopituitarism was hypothesized and a brain MRI was performed. An empty sella (with a flattened pituitary gland), without focal lesions was shown (figure 1).
On ultrasound the patient had a slightly enlarged and heterogeneous thyroid, with bilateral hypoechogenic nodules (the largest of which with 7.8 x 5.1 x .51mm).
These results led to secondary adrenal insufficiency, hypogonadotrophic hypogonadism and presumable primary autoimmune hypothyroidism diagnosis. The patient was started on hormonal replacement therapy with prednisolone 5 mg/day and with levothyroxine 0.025 mg/day, changed to 0,075mg 4 days later. He was discharged at the 8thday with sodium levels of 128 mmol/L. His medication was switched to valsartan and amlodipine.
In the follow-up visit, 3 weeks latter, the patient was felling well but still with low stamina, described by the family as usual. He had normal sodium levels (135mmol/L). After obtaining normal levels of prostatic specific antigen (PSA) and an unremarkable prostatic ultrasound, testosterone decanoate was also initiated (250 mg every 4 weeks). A month later the patient was symptom free (referring no somnolence or lethargy), and sodium levels were within the normal range (135 mmol/L), so prednisolone was titrated to 2.5 mg/day. One year later, due to a rise in PSA levels, testosterone decanoate was suspended. The patient remained asymptomatic medicated with prednisolone 2,5mg and levothyroxine 0,075mg.
DISCUSSION
Hyponatremia is mostly a disorder of water balance, with a relative excess of body water.1Sodium and its accompanying anions are the major effective plasma solutes. Therefore, hyponatremia and hypo-osmolality are usually synonymous.1The patient had a reduced plasma osmolality, normal lipids and protein profile, excluding pseudohyponatremia.
Cortisol usually suppresses production of corticotrophin-releasing hormone and antidiuretic hormone (ADH). Thus, in secondary adrenal insufficiency, cortisol fails to suppress ADH resulting in hyponatremia (impaired free water excretion).1Isolated cortisol deficiency due to hypopituitarism (impaired ACTH secretion) has intact aldosterone production, preserving the extracellular fluid volume, maintaining euvolemia (as opposed to mineralocorticoid deficiency in primary adrenal insufficiency). Therefore, replacement with isotonic saline does not reverse impaired water excretion (and hyponatremia) in secondary adrenal insufficiency, as mentioned in the case description. This could also be the explanation to why hypotension is less prominent in secondary adrenal insufficiency and why in this case the patient was not hypotensive. The fact that aldosterone production is not affected also explains the absence of hyperkalemia.5
ADH is not measured routinely and urine osmolality can be used as a surrogate marker for its action1. As mentioned before, hypocortisolism causes inappropriate ADH secretion. Hypothyroidism is believed to cause hyponatremia by reduced cardiac output (stimulating ADH secretion) and by decreasing glomerular filtration rate.6In this case, and although the urine osmolality is inappropriate elevated (>100mosm/L), we expected a higher urinary osmolality because in case of non-suppressed ADH activity, urine osmolality usually exceeds serum osmolality1. For urine osmolarities between 100 mOsm/L and the value of the serum osmolality (as in this case) excessive fluid intake may outweigh moderately suppressed vasopressin activity1.
The diagnosis of adrenal insufficiency is usually based on low morning cortisol and confirmed with a functional test.7The most used is the cosyntropin test (also named Synacthen ®) that consists in the measurement of cortisol after the administration of this peptide (that stimulates the adrenal cortex as ACTH does).8If cortisol remains low, adrenal insufficiency is present and if ACTH is also low, the diagnosis of secondary adrenal insufficiency is made. 6The cosyntropin stimulation test has a lower sensitivity in secondary adrenal insufficiency8: in patients with mild insufficiency or of recent onset the test may be normal6. So, it is helpful for ruling it in, but not for ruling it out. Unfortunately, this test is not available in our institution and so we weren’t able to use it in this patient work-up.
Cortisol counters the action of insulin, so in adrenal failure hypoglycaemia can occur because cortisol deficiency promotes fasting hypoglycaemia. In some individuals it only occurs under stress situations.9,10In this patient no episode of hypoglycaemia was detected.
In this case, the elevated TSH and low free T4 levels suggest the diagnosis of primary hypothyroidism, possibly autoimmune thyroiditis (according to the presence of thyroid peroxidase [TPO] and thyroglobulin [Tg] antibodies). Some authors have reported the high prevalence (40%) of primary thyroid dysfunction (with autoimmunity in a third of them), raising the hypothesis of an autoimmune process involving the pituitary gland.11In secondary hypothyroidism TSH levels may be low, normal, or slightly elevated, and don’t correlate with free T4 levels, which can make the distinction between primary and secondary hypothyroidism more difficult.2Hypothyroidism can be a contributor to the development of hyponatremia, but low sodium is only seen in severe cases or in myxedema.12Furthermore, when hyponatremia accompanies hypopituitarism, it is generally a manifestation of secondary adrenal insufficiency, rather than coexisting hypothyroidism.3Hence, regardless of its aetiology, it is unlikely that the reduced thyroid function can, by itself, justify the severe hyponatremia. In this case we considered it to be an adjoining causal factor.
The patient was also on hydrochlorothiazide, and albeit this was not the main cause of the hyponatremia, it acted as a possible contributor (by stimulating ADH production)1, so it was replaced by amlodipine.
In empty sella syndrome, inadequate glandular secretion can be caused by the compression of the pituitary parenchyma with a stretched pituitary stalk or by derangements in the neurotransmitters related to cerebrospinal fluid pressure.4This disturbance can be of different degrees: panhypopituitarism, combined or isolated hormonal deficit.4Prolactin can be elevated, within normal values or in deficit.4Replacement hormone therapy must have an appropriate temporal sequence, starting with corticosteroids before levothyroxine to avoid adrenal crisis.6In our patient the replacement was started simultaneous, but with a low dose of levothyroxine, that could justify the patient’s stability. Sexual hormones should only be introduced when the patient condition is stabilized.4Hydrocortisone it’s the most recommended corticosteroid and is usually used in divided doses, but longer-acting glucocorticoids as prednisolone can be used in selected cases.2In this particular case, prednisolone was used to increase adherence, as there was a risk of non-compliance with drugs taken outside morning schedule.
Sexual hormones replacement is more controversial, but the Endocrine Society recommends testosterone replacement in order to prevent anaemia, reduce fat mass, improve bone mineral density, libido, sexual function, energy levels, sense of well being and muscle mass and strength.1It is important to remember however that studies in this area have used different testosterone formulations and had short follow-ups.1
This case portraits the rare presentation of hyponatremia as the first sign of hypopituitarism, showing the relevance of investigating this electrolyte disorder since its treatment depends on its correct diagnosis.
Quadro I
Laboratory results
| Parameter | Value | Reference range |
| | | |
| Hemoglobin | 12.8 g/dL | (13.5 - 17-5) |
| Urea | 28 mg/dL | (18 - 55) |
| Creatinine | 0.87 mg/dL | (0.7 – 1.3) |
| Sodium | 108 mmol/L | (136 - 145) |
| Potassium | 4.4 mmol/L | (3.5 – 5.1) |
| Glucose | 84 mg/dL | (74 - 106) |
| Serum osmolarity | 220 mosmol/L | (280-300) |
| Urine osmolarity | 146 mosmol/L | (300 – 900) |
| Urinary sodium | 27 mmol/L | (<20) |
| Adrenocorticotropic Hormone (ACTH) in the morning | 8.2 pg/mL | (3.6 - 60.5) |
| Serum Cortisol in the morning | 5.43 µg/dL | (5,27 - 22,45) |
| Thyroid Stimulating Hormone (TSH) | 16.56 mUI/L | (0.55 - 4.78) |
| Free Thyroxine (FT4) | 2.2 pmol/L | (10.6 - 19.4) |
| Antibodies to thyroid peroxidase (TPO) | 870 UI/mL | < 60.0 |
| Antibodies to thyroglobulin (Tg) | 244 UI/mL | < 114 IU/mL |
| Follicle-stimulating hormone (FSH) | 3.2 mUI/mL | (1.4 - 18.1) |
| Luteinizing hormone (LH) | 1.3 mUI/mL | (1.5 - 9.3) |
| Prolactin | 2.0 ng/mL | (2.1 - 17.7) |
| Testosterone | 121 ng/dL | (193.0 - 740.0) |
| Insulin-like growth factor-1 (IGF-1) | 27ng/mL | (27 - 246) |
| Growth hormone (GH) | <0.05 ng/mL | (< 2,47) |
Laboratory results
Figura I
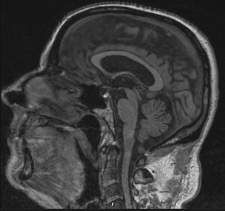
Brain MRI showing an empty sella (with a flattened pituitary gland).
BIBLIOGRAFIA
1.Spasovski G, Vanholder R, Allolio B, Annane D, Ball S et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia.Eur J Endocrinol. 2014;170(3): G1-47.
2.Fleseriu M, Hashim I, Karavitaki N, Melmed S, Murad M et al. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline.J Clin Endocrinol Metab 2016;101(11): 3888–3921.
3.Catalano A, Basile G, Ferro C, Scarcella C, Bellone F et al. Hyponatremia as a leading sign of hypopituitarism.J Clin Transl Endocrinol: Case Rep.2017;5:1-3.
4.Chiloiro S, Giampietro A, Bianchi A, Tartaglione T, Capobianco A, et al. Diagnosis of Endocrine Disease: Primary empty sella: a comprehensive review.Eur J Endocrinol. 2017;177(6): R275-R285.
5.Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia Treatment Guidelines 2007: Expert Panel Recommendations.Am J Med. 2007;120(suppl 1): S1-S21.
6.Liamis G, Milionis HJ, Elisaf M. Endocrine disorders: causes of hyponatremia not to neglect.Ann Med. 2011;43(3): 179-187.
7.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, et al.Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline.J Clin Endocrinol Metab. 2016;101: 364-389.
8.Hamilton DD, Cotton BA. Cosyntropin as a diagnostic agent in the screening of patients for adrenocortical insufficiency.Clin Pharmacol.2010;2:77-82.
9.Diéguez Felechosa M, Valdés Gallego N, García-Alcalde Fernández ML, Casal Alvarez F. Hipoglucemia como manifestación de insuficiencia suprarrenal iatrógena por esteroides tópicos.Endocrinol Nutr.2013;60: e21-e22.
10.Oprea A, Bonnet NCG, Pollé O, Lysy PA. Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency.Ther Adv Endocrinol Metab. 2019;10:1–27
11.Del Monte P, Foppiani L, Cafferata C, Marugo A, Barnasconi D. Primary “empty sella” in adults: endocrine findings.Endocr J. 2006;53: 803-809.
12.Itoh F, Hozawa N, Branch J. A Pitfall in the Differential Diagnosis of Hyponatremia.General Medicine. 2015;16(2): 107-112.


