CASE DESCRIPTION
A 34-year-old Chinese pregnant woman (26 weeks’ gestation) presented to the Emergency Department with acute epigastric pain. Previous pregnancy with C-section at 32 weeks’ gestation, which she said was caused by “excess fat in the blood”. She had no follow-up since then and was on no medication.
On admission she had abdominal tenderness in the upper quadrants. The lab results are in Table 1. We highlight serum lipase 1786 U/L and serum triglyceride (TG) 5036 mg/dL (Fig.s 1 and 2). The abdominal ultrasound showed pancreatic oedema and peri-pancreatic effusion.
She was admitted to our intensive care unit (ICU), hemodynamically stable, and started centrifugal TPE, considering extremely elevated levels of TG, gestational age, presence of a serious complication, risk of pre-term labour and risk of foetal and maternal death. Each TPE session lasted 119 to 221 minutes. The volume corresponded to 1 ± 0.27 plasma volume, requiring 7 to 12 cycles. She became asymptomatic once TG were controlled (500-700 mg/dl) and experienced transient tachycardia and hypotension during treatment. She started low lipid diet and omega-3 supplementation. Foetal ultrasound revealed a foetus (1109g) with good vitality and growth.
Since TG raised after each plasmapheresis, after reviewing the literature, it was decided to start 200mg fenofibrate per day.
On day 9 (27 weeks + 2 days), she complained of newly onset abdominal pain. Obstetric re-evaluation was normal. A new abdominal ultrasound revealed worsening of the peri-pancreatic effusion, and an MRI showed pancreatic necrosis (Fig.s 3 and 4).
The patient remained without fever, abdominal pain, or evidence of infection (negative blood cultures and low levels of c-reactive protein). Plasmapheresis was continued for 19 sessions (Figure 5), and subsequent abdominal ultrasounds showed no worsening.
At 31 weeks and 6 days gestation, after foetal lung maturation with betamethasone, she had an elective C-section and delivered a male sex new-born (1746g, Apgar score: 9/10/10).
Despite low molecular weight heparin (LMWH) prophylaxis during ICU stay, the patient developed a deep venous thrombosis associated to the TPE catheter and was started on anticoagulation with LMWH. Genetic thrombophilia study was negative.
She was discharged 11 days after delivery with follow-up in outpatient clinic of Endocrinology, Transfusion Medicine and Internal Medicine. After 9 months of fenofibrate therapy: TG 766mg/dL, total cholesterol 265mg/dL, HDL 31mg/dL, LDL 106mg/dL.
A genetic and molecular study for familiar dyslipidaemias revealed a heterozygotic mutation in LPL gene. As this doesn’t justify the phenotype, the study of rare familial dyslipidaemias (monogenic causes) is still ongoing.
DISCUSSION
Acute pancreatitis in pregnancy is rare (1:1000-12000), but potentially life-threatening.1. It increases from 19% in the first trimester to 53% in the third trimester.2As in the general population, the most common causes of AP include gallstone disease, followed by hypertriglyceridemia.3, 4, 5,9
The increase of plasma TG is essential to ensure normal foetal development, and its levels are usually under 300 mg/dL. This occurs mainly in the third trimester and is due to high oestrogen levels.3
Mechanisms behind the development of AP due to hypertriglyceridemia remain unknown. One of the most accepted theories suggests that high levels of TG cause high serum viscosity, activate pancreatic lipase, leading to the rise in free fatty acids and chylomicrons, inducing direct toxicity to acinar cells, ischemia and inflammation.
Severe gestational hypertriglyceridemia is a rare condition defined as plasma TG > 1000mg/dL, and it occurs mainly in the presence of genetic mutations. Depending on the type of familial lipid disorders, patients may present different phenotypes. In this case, it was only identified a heterozygotic change in LPL gene which does not justify the clinical presentation. Notwithstanding this, it is important to rule out predisposing factors as insulin-resistance, hypothyroidism, nephrotic syndrome, glucocorticoids, beta-blockers, protease inhibitors and alcohol, which were all absent.6
Severe gestational hypertriglyceridemia can induce AP, with mortality rates between 7.5 and 21.0% (mother) and between 20 and 50% (foetus).2,6,12 Luo et al performed a review, confirming hypertriglyceridemia as its second leading cause, demonstrating the higher risk of pancreatic abscesses or pseudocysts.8 Furthermore, it can also cause pre-eclampsia, hyperviscosity syndrome,3,13 foetal macrosomia and pre-term labour.14 Therefore, it is essential to establish a timely therapeutic strategy, avoiding complications.
Treatment for AP induced by gestational hypertriglyceridemia consists of supportive measures and reduction of TG.6, 10, 11,15 Bertha Wong et al. provided a practical approach to severe hypertriglyceridemia.6 including: low–fat diet, omega-3 fatty acids, lipid-lowering therapy (after the first trimester), insulin and plasmapheresis (Fig. 6). We acted according to this approach and started fenofibrate, which revealed to be safe.
The American Society for Apheresis classifies TPE as a Category III (individualized decision) and Grade 2C (weak recommendation).7,16 However, TPE should be considered as first therapeutic choice if: a) failure of dietetic and pharmacological measures, b) plasma TG> 1000 mg/dL, c) plasma lipase > 3-fold the normal upper limit, d) hypocalcaemia, e) lactic acidosis and f) organ dysfunction or clinical worsening.2 Our patient met 2 of these 6 criteria. Regarding efficacy, in a retrospective study, Huang et al,15 observed significant reduction of TG levels, SIRS criteria and length of stay.
Centrifugal TPE appears to be superior to double membrane filtration TPE as the triglycerides tend to clog the filters.16 Considering the choice of replacement fluid, donor plasma can replace lacking enzymes for the metabolism of lipids.17 However, the use of human blood components always carries some risks, and concerning pregnancy, alloimmunization cannot be overlooked, so we used 5% albumin instead. Regarding anticoagulation, we chose citrate which is associated with lower mortality. The use of citrate requires special care concerning hypocalcaemia, stabilized using calcium gluconate.16,18
This case reports a rare clinical condition presenting a life-threatening complication, in which TPE proved to be an effective and safe therapy.
As a conclusion, despite the absence of randomized clinical trials, there is clinical evidence that leads us to strongly recommend the use of plasmapheresis in severe cases of pregnancy-induced hypertriglyceridemia, particularly in cases where there is very high risk of potential complications.
- ACKNOWLEDGMENT
- The authors would like to acknowledge Dr. Lia Lêdo for her collaboration in the treatment of this patient and her support in the development of this manuscript.
Figura I
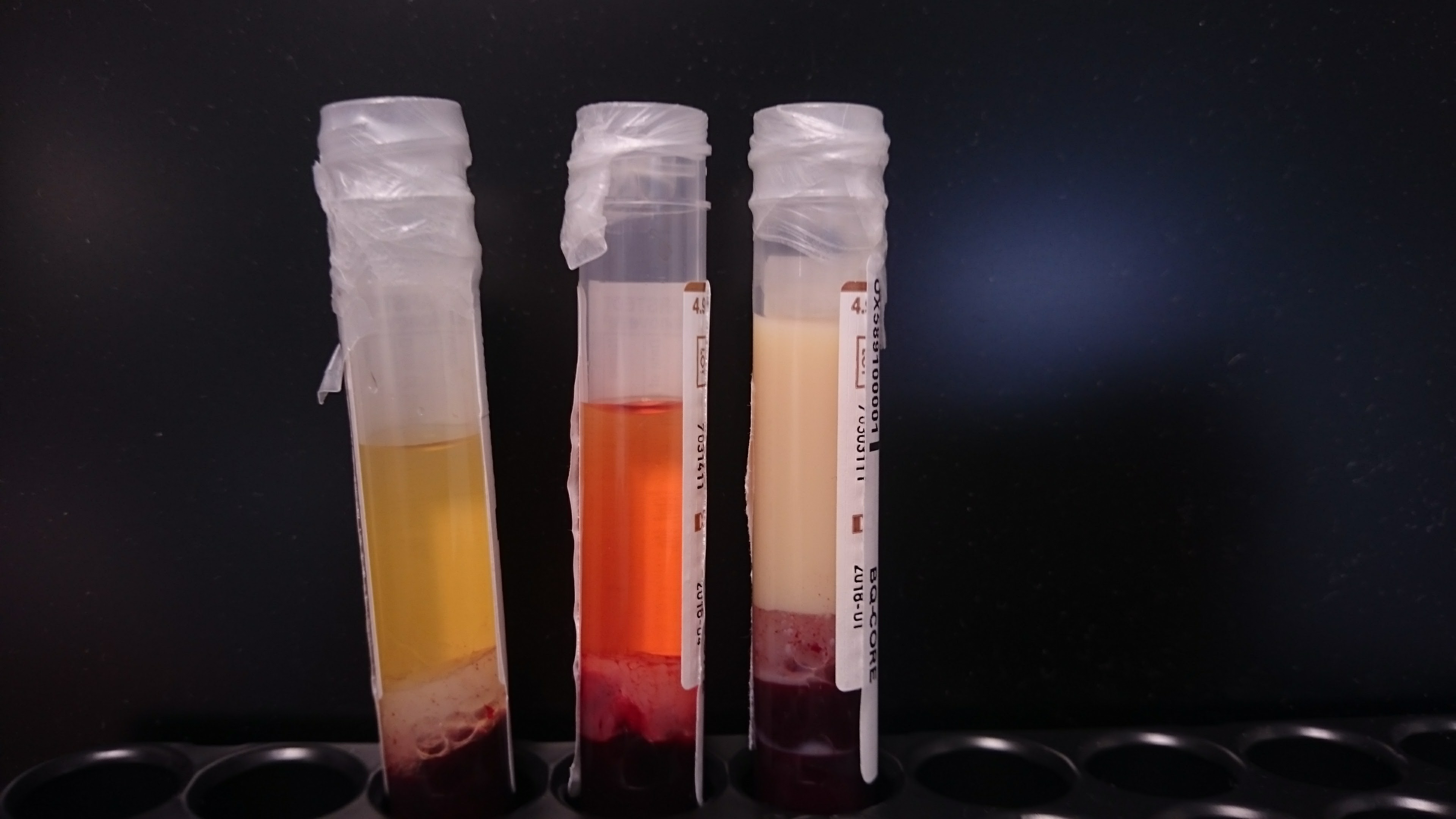
Blood samples drawn from the patient, before the beginning of therapeutic plasma exchange, showing high concentration of triglycerides.
Figura II
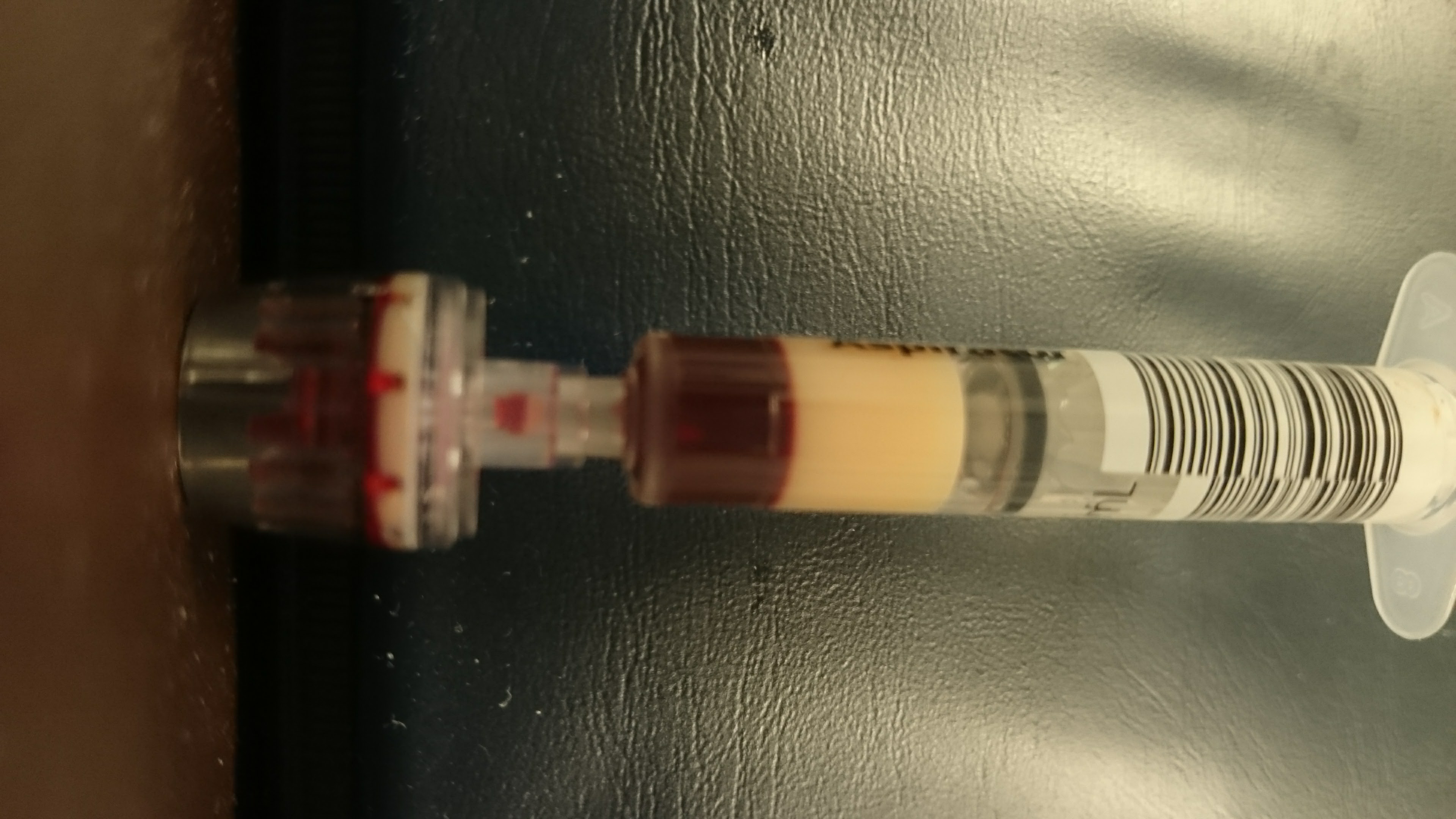
Blood samples drawn from the patient, before the beginning of therapeutic plasma exchange, showing high concentration of triglycerides.
Figura III
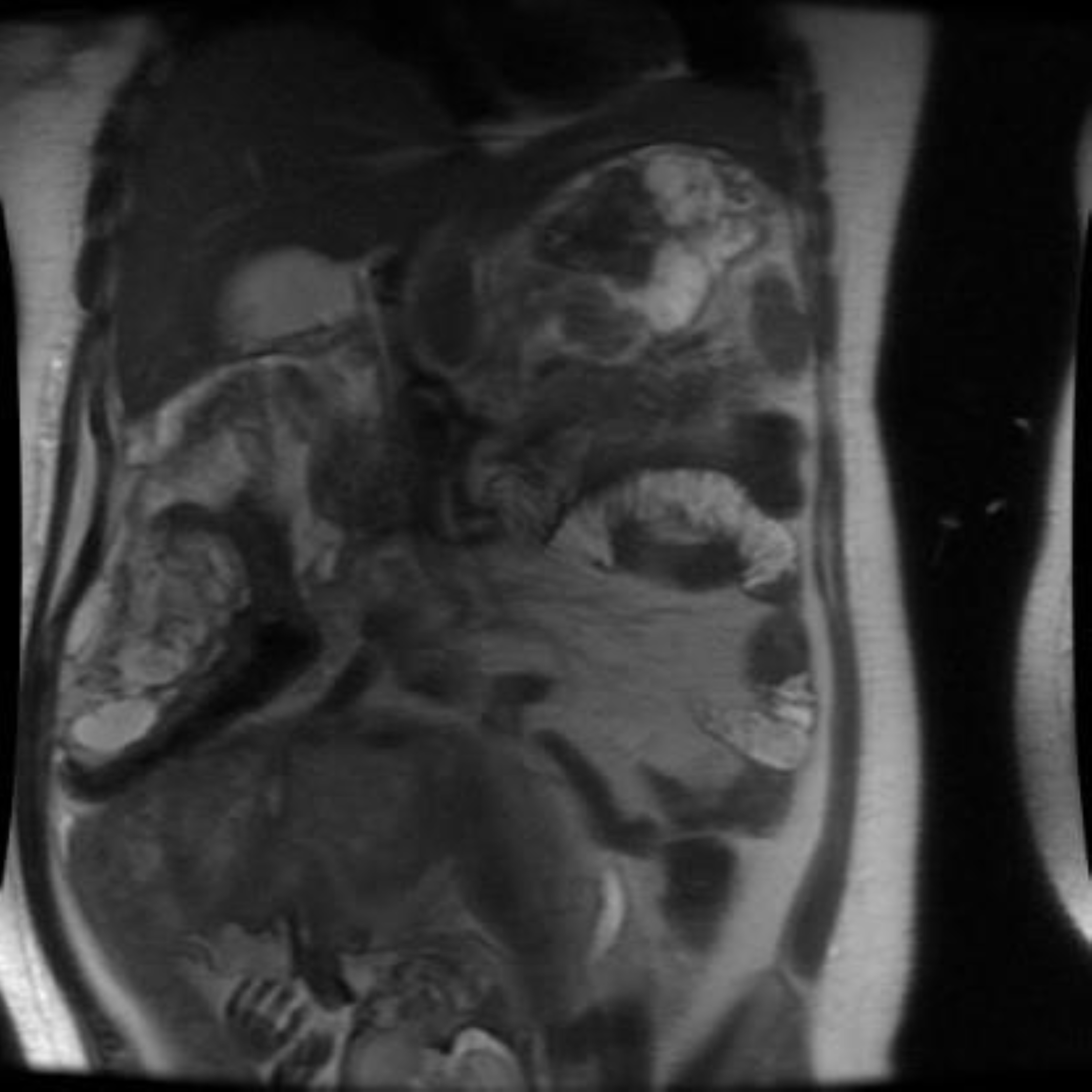
Superior abdominal MRI (left coronal, right axial), that suggests extra pancreatic necrosis.
Figura IV
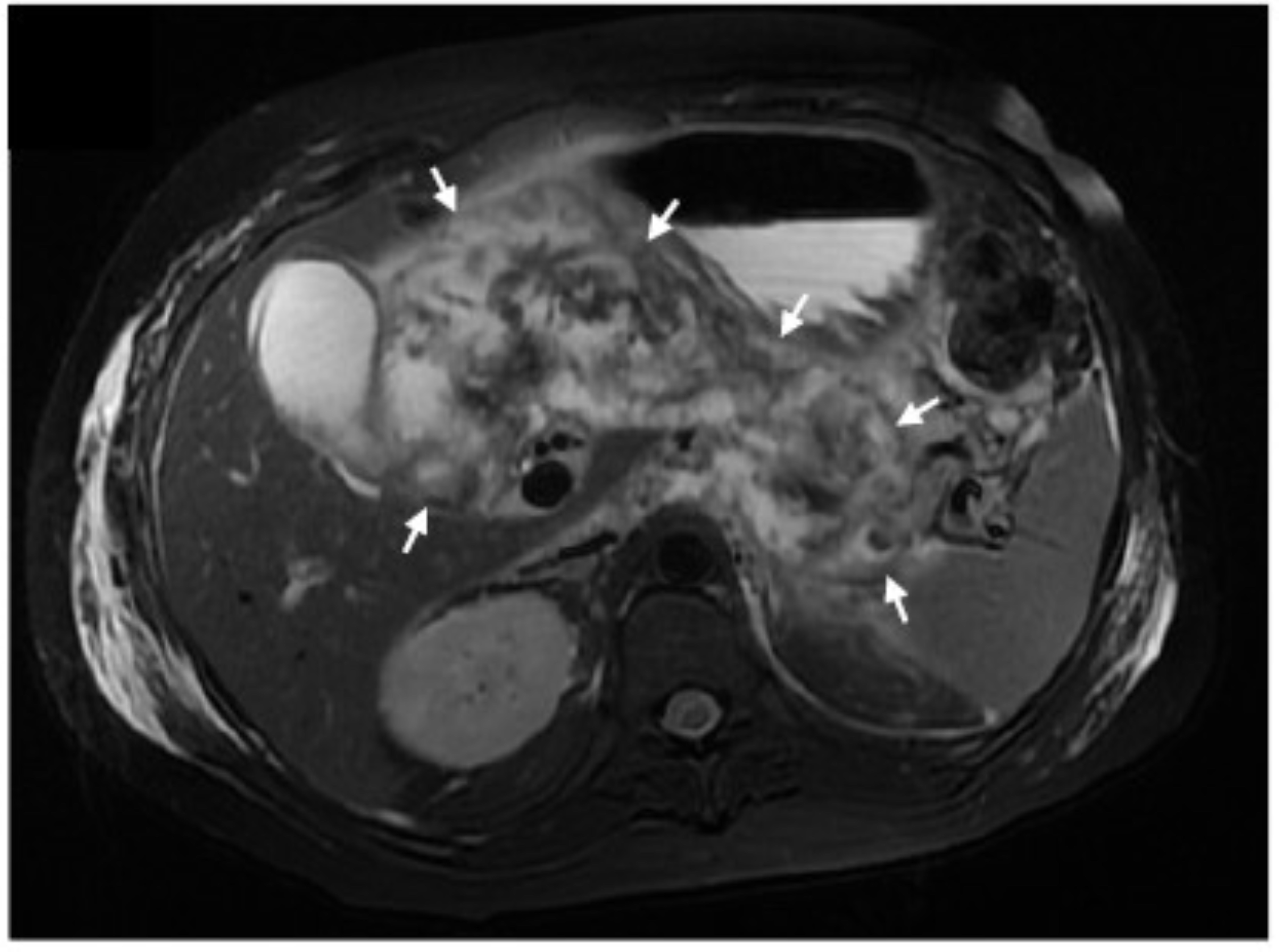
Superior abdominal MRI (left coronal, right axial), that suggests extra pancreatic necrosis.
Figura V
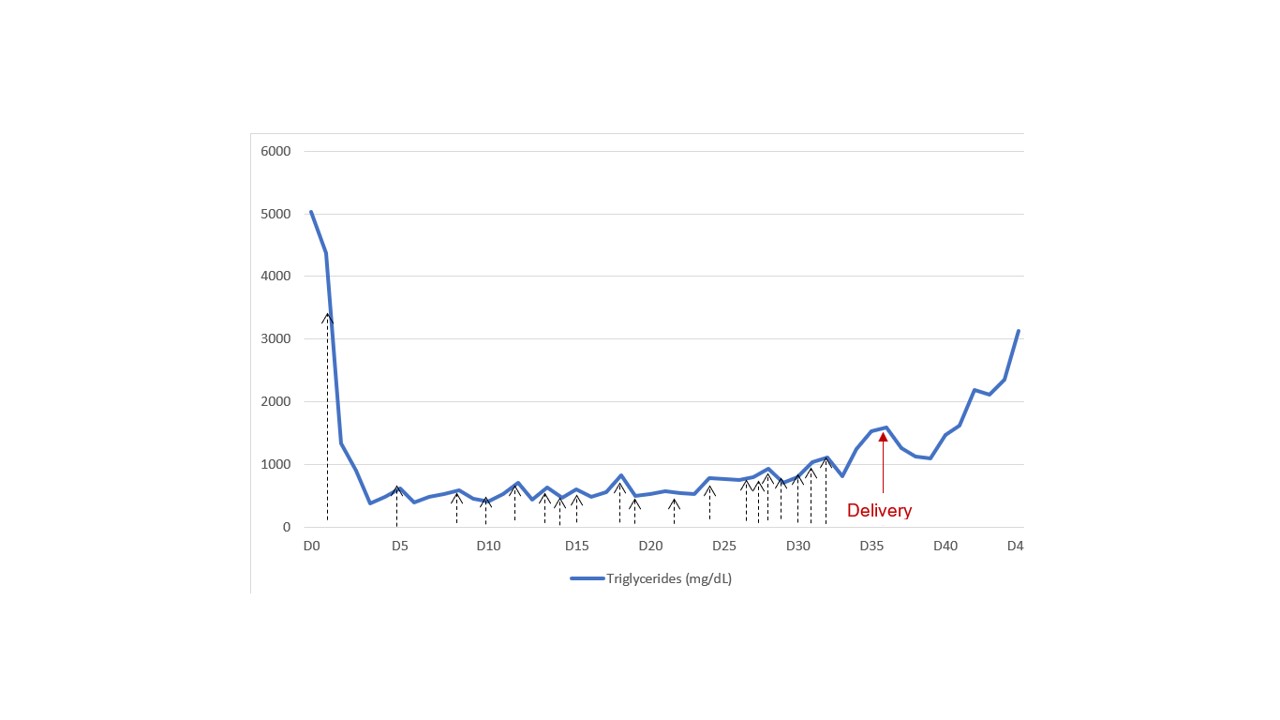
Triglyceride values during hospitalization. The black arrows correspond to plasmapheresis sessions (N=19), the red arrow correspond to the delivery day.
Figura VI
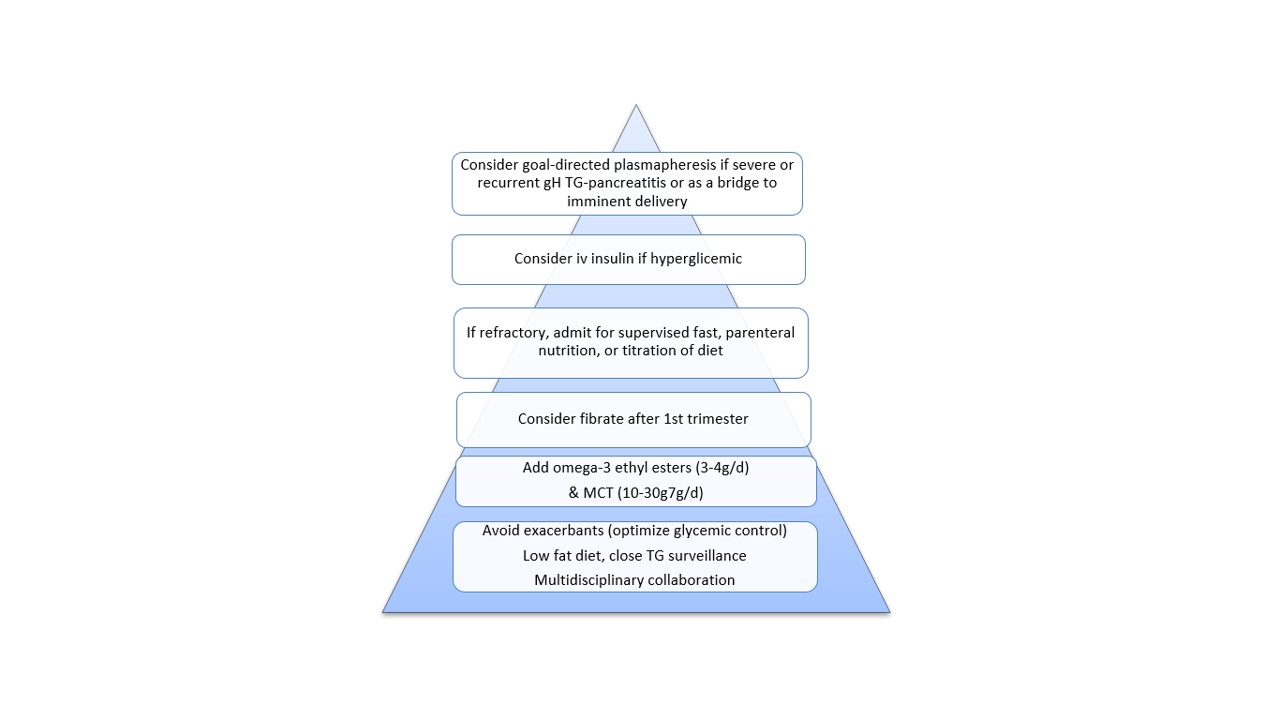
Recommended evidence-based approach to severe gestational hypertriglyceridemia treatment (according to Wong et al [6])
BIBLIOGRAFIA
1. Geng, Y., et al., Severe acute pancreatitis during pregnancy: eleven years experience from a surgical intensive care unit. Dig Dis Sci, 2011. 56(12): p. 3672-7.
2.Gupta, N., et al., Severe hypertriglyceridemia induced pancreatitis in pregnancy. Case Rep Obstet Gynecol, 2014. 2014: p. 485493.
3.Goldberg, A.S. and R.A. Hegele, Severe hypertriglyceridemia in pregnancy. J Clin Endocrinol Metab, 2012. 97(8): p. 2589-96.
4. Manas Garcia, M.D., et al., Hipertriglyceridemia induced acute pancreatitis in pregnancy. Clin Investig Arterioscler, 2017. 29(6): p. 275-7.
5. Pitchumoni, C.S. and B. Yegneswaran, Acute pancreatitis in pregnancy. World J Gastroenterol, 2009. 15(45): p. 5641-6.
6. Wong, B., T.C. Ooi, and E. Keely, Severe gestational hypertriglyceridemia: A practical approach for clinicians. Obstet Med, 2015. 8(4): p. 158-67.
7. Basar, R., et al., Therapeutic apheresis for severe hypertriglyceridemia in pregnancy. Arch Gynecol Obstet, 2013. 287(5): p. 839-43.
8. Safi, F., et al., Management of familial hypertriglyceridemia-induced pancreatitis during pregnancy with therapeutic plasma exchange: a case report and review of literature. Am J Ther, 2014. 21(5): p. e134-6.
9. Luo, L., et al., Clinical characteristics of acute pancreatitis in pregnancy: experience based on 121 cases. Arch Gynecol Obstet, 2018. 297(2): p. 333-9.
10. Papadakis, E.P., et al., Acute pancreatitis in pregnancy: an overview. Eur J Obstet Gynecol Reprod Biol, 2011. 159(2): p. 261-6.
11. Cain, M.A., et al., Gallstone and Severe Hypertriglyceride-Induced Pancreatitis in Pregnancy. Obstet Gynecol Surv, 2015. 70(9): p. 577-83.
12. Lim, R., S.J. Rodger, and T.L. Hawkins, Presentation and management of acute hypertriglyceridemic pancreatitis in pregnancy: A case report. Obstet Med, 2015. 8(4): p. 200-3.
13. Gallos, I.D., et al., Pre-eclampsia is associated with, and preceded by, hypertriglyceridaemia: a meta-analysis. Bjog, 2013. 120(11): p. 1321-32.
14. Tang, S.J., et al., Acute pancreatitis during pregnancy. Clin Gastroenterol Hepatol, 2010. 8(1): p. 85-90.
15. Huang, C., et al., Clinical features and treatment of hypertriglyceridemia-induced acute pancreatitis during pregnancy: A retrospective study. J Clin Apher, 2016. 31(6): p. 571-8.
16. Schwartz, J., et al., Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher, 2016. 31(3): p. 149-62.
17. Cunha, C., et al., Plasma exchange in hypertriglyceridaemic acute pancreatitis: case report. Portuguese Journal of Nephrology & Hypertension, 2016. 30: p. 140-4.
18. Denker, E.S.a.B.M., Plasmapheresis, in Brenner and Rector´s The Kidney, G.M.C. Karl Skorecki, Philip A. Marsden, Maarten W. Taal, Alan S. L. Yu, Editor. 2016, Elsevier: Philadelphia. p. 2148-65.







