INTRODUCTION
Gram-negative bacilli such as Pseudomonas aeruginosa are a rare cause of meningitis and ventriculitis, although the incidence of Gram-negative bacilli meningitis seems to be increasing.1 Patients who develop this central nervous system (CNS) infection most commonly have a history of neurosurgical procedures, including extraventricular drainage (EVD) or shunt insertion.2 Bacterial meningitis has an estimated incidence of 4–6 cases per 100,000 adults per year in developed countries, being the most frequent etiologic agents in adults Streptococcus pneumoniae and Neisseria meningitidis.3 Spontaneous meningitis caused by gram-negative bacilli, besides Haemophilus influenzae, is uncommon and poorly characterized as studies combine patients with spontaneous meningitis and meningitis secondary to trauma or neurosurgery, whose characteristics are specific and differentiating.4 Exclusive data regarding ventriculitis is even more scarce, so conclusions are frequently inferred from patients with meningitis. High risk groups include the elderly, patients with nosocomial infection regarding other foci and neurosurgical patients.5 There is a notorious lack of information in immunocompromised patients. Herein we report a case of Pseudomonas aeruginosa ventriculitis occurred in 55-year-old men, who had a heart transplantation 5 months earlier, without immediate complications. He was admitted at the emergency department with nonspecific complains.
CASE PRESENTATION
A 50-year-old male was admitted to the Intensive Care Unit (ICU) for septic shock due to pneumonia, requiring invasive mechanical ventilation and aminergic support upon admission on the emergency department. Three days before, he started complaining of shortness of breath and abdominal pain, without fever; besides dizziness and blurred vision, no other neurological signs or symptoms were reported, namely neck stiffness or photophobia.
His past medical history was significant for heart transplantation 3 months before, due to ethylic dilated cardiomyopathy, without major immediate intercurrences. He was kept on an immunosuppressive regimen with tacrolimus 2mg b.i.d, prednisolone 10mg b.i.d and mycophenolate mofetil 1000mg b.i.d.
As he met sepsis criteria, broad-spectrum antimicrobial coverage was initiated with meropenem (2gm IV q8h). On admission, relevant findings were a C-reactive protein of 33.25mg/dl (reference range: 0-0.5 mg/dl), white blood cell count of 19.8 x 109 /L (reference range: 4-10 x 109 /L), neutrophils of 16.4 x 109 /L (reference range: 2-7 x 109 /L). Chest radiograph showed middle lobe consolidation of right lung with interstitial infiltrates (Figure 1).
Figure 1 Chest radiograph with middle lobe consolidation of right lung with interstitial infiltrates.
P. aeruginosa was isolated in the first two sets of blood cultures, with susceptibility to ceftazidime and gentamycin and later, in endotracheal aspirate with the same resistance profile; as susceptibility to meropenem was not confirmed in both antibiotic susceptibility testing, ceftazidime (2gm IV q8h) and gentamicin (7mg/kg q24h) were initiated. After improvement of respiratory failure and suspension of sedo-analgesia, the patient maintained an altered state of consciousness (3 points in Glasgow Coma Scale). Acute cerebrovascular events and metabolic causes of coma were meticulously excluded. Electroencephalogram revealed severe diffuse encephalopathy. This finding was further explored with serial neuroimaging with cerebral computerized tomography that showed intra-ventricular purulent collection with linear ependymal enhancement, confirming ventriculitis (Figure 2).
Figure 2 Cerebral CT showed, besides areas of ischemia, intra-ventricular purulent collection with linear ependymal enhancement, confirming ventriculitis.
In this context, an extraventricular drain was placed revealing a densely cellular cerebrospinal fluid (leucocytes of 8000/mm3), with predominance of polymorphonuclear, glycorrhachia of 3mg/dl (range 40-70) and proteinorrhachia of 237mg/dl (range 15- 40) and with culture positive for P. aeruginosa susceptible only to gentamycin and colistin. Intrathecal gentamicin (8mg, q.d) and colistin (5-10mg q.d) were associated to previous intravenous antibiotics.
Besides invasive mechanical ventilation, the patient also benefited from vasoactive amines at an early stage. He was also treated with intravenous dexamethasone (4mg b.i.d) to minimize vasogenic oedema resulting from the cerebral inflammatory process as well as levetiracetam IV 1000mg, b.i.d. Immunosuppressants above referred were replaced by cyclosporine continuous perfusion. Nutritional support was ensured by enteric nutrition bag with adequate calorie and protein requirements considering the high catabolic state due to severe infection.
Despite optimized therapeutic measures, the outcome was fatal, and the patient died 13 days after ICU admission.
DISCUSSION
P. aeruginosa is an uncommon agent of meningitis and ventriculitis and is associated with significant morbidity and mortality.6,7 Ventriculitis due to P. aeruginosa is mostly described as resulting from infections in the context of surgical manipulation of the CNS as, for example, in the placement of ventricular drains but also by wounds.8,9
Case series reported in the literature have shown that this agent is responsible for 1–18% of nosocomial meningitis cases. Pointed risk factors, besides surgery, include prolonged hospital stay and the use of broad-spectrum antibiotics.10 The recorded mortality is often high, due to treatment failure and relapses, reaching 80% in some series.
In fact, susceptibility profile of P. aeruginosa is concerning, and treatment options can be limited, also due to the poor penetration of many antimicrobial agents into the CSF.11 Antibiotics are usually restricted to intravenous (IV) drugs such as ceftazidime, carbapenems (meropenem and imipenem), aminoglycosides (gentamicin, amikacin or tobramycin) and ciprofloxacin, often combined with intrathecal aminoglycosides or colistin, with long treatment periods (from 14 to 28 days).12,13
A recent case series reported that 19 out of 21 patients who grew P. aeruginosa from cerebrospinal fluid culture had previously undergone a neurosurgical procedure or had extraventricular devices.14 More than half had P. aeruginosa isolated from another site previously. In a report of spontaneous bacterial meningitis, Gram-negative bacilli were detected in only 7% of cases, being the most common pathogens Escherichia coli and Pseudomonas species.15 The same study found that nosocomial acquisition of infection, urinary tract infection, advanced age, acute respiratory failure, hypotension, and history of cancer were conditions associated with Gram-negative bacilli meningitis.
This case report intends to emphasize several important messages. First, in the presence of an unexplained mental status deterioration, the workup must remain broad. As occurred in the present case, even though there was an identified infection source, it is crucial to keep in mind the hypothesis of a rarer intracerebral infection as it requires different management, including urgent evaluation of antibiotic selection and dosing to ensure CNS penetration as well as neurosurgical evaluation and orientation. Second, in a patient under immunosuppressive therapy, infectious focus is often atypical, and clinical manifestations are commonly nonspecific. Finally, use of intrathecal antibiotics in association with intravenous formulation should be considered as soon as possible to ensure the highest concentration at infection site, particularly within brain ventricles.16 Prevalence of resistance to third generation cephalosporins and carbapenems is a worrying development.17,18 Detailed surveillance of local pathogens and resistance patterns is essential to guide empirical therapy as resistant microorganisms to both antibiotic classes is a distressing and increasingly prevalent reality, as there are few therapeutic available options.
Figura I
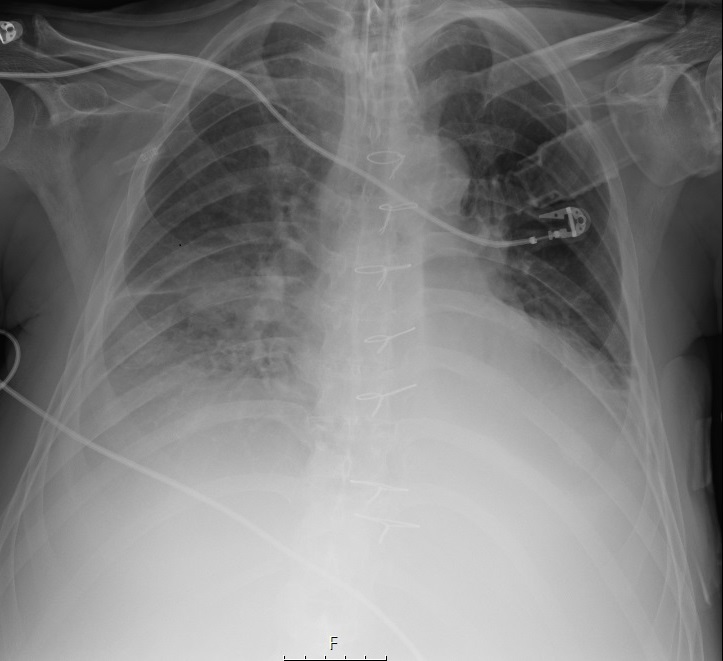
Chest radiograph with middle lobe consolidation of right lung with interstitial infiltrates.
Figura II
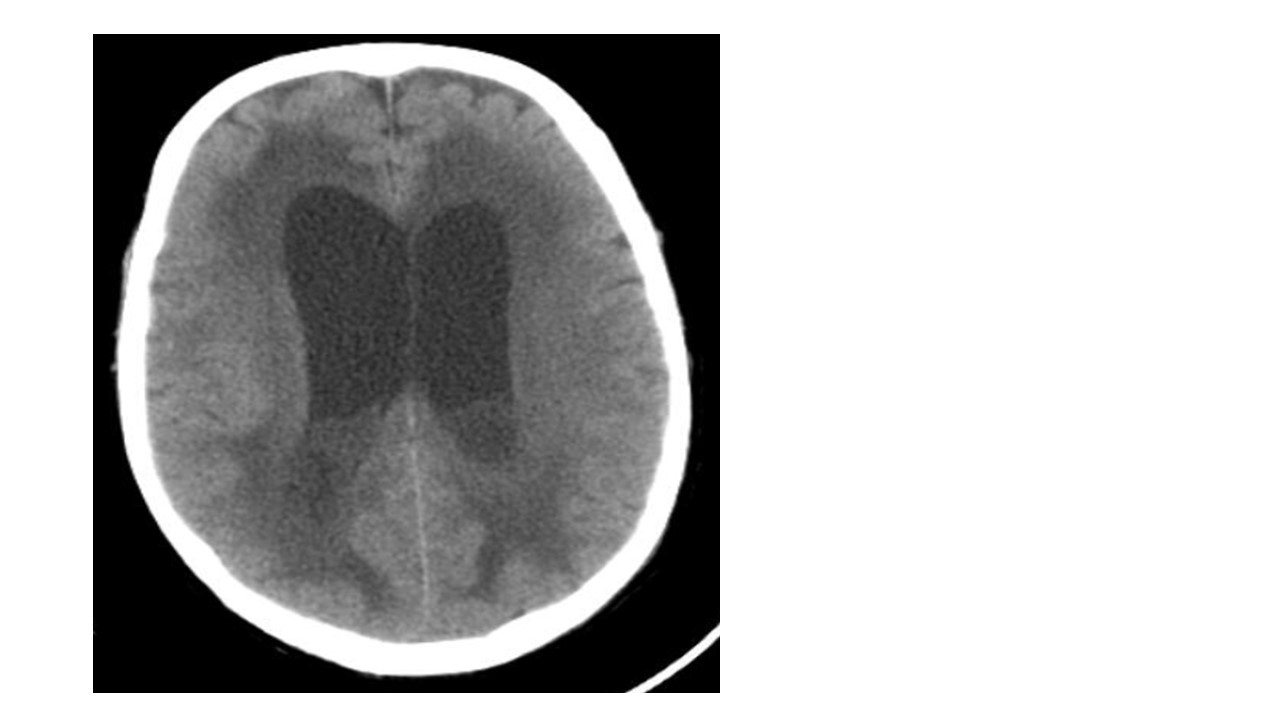
Cerebral CT showing, besides areas of ischemia, intra-ventricular purulent collection with linear ependymal enhancement, confirming ventriculitis.
BIBLIOGRAFIA
1. Busl KM, Bleck TP. Bacterial Infections of the Central Nervous System. Curr Infect Dis Rep 2013; 15, 612–30.
2. Pomar V, Benito N, López-Contreras J, et al. Spontaneous gram-negative bacillary meningitis in adult patients: characteristics and outcome. BMC Infect Dis 2013; 13:451.
3. Ramanan M, Lipman J, Shorr A, et al. A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect Dis 2015; 15:3
4. Huang CR, Lien, CY, Tsai, WC, et al. The clinical characteristics of adult bacterial meningitis caused by non-Pseudomonas (Ps.) aeruginosa Pseudomonas species: A clinical comparison with Ps. aeruginosa meningitis. The Kaohsiung Journal of Medical Sciences2018; 34: 49-55.
5. Federico G, Tumbarello M, Spanu T et al. Risk factors and prognostic indicators of bacterial meningitis in a cohort of 3580 postneurosurgical patients. Scand J Infect Dis 2001; 33:533–7.
6. Beer R, Lackner P, Pfausler B, et al. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol 2008; 255(11):1617–24.
7. Gallaher C, Norman J, Singh A, et al. Community-acquired Pseudomonas aeruginosa meningitis. Case Reports 2017; bcr-2017-221839.
8. Tängdén T, Enblad P, Ullberg M, et al., Neurosurgical Gram-Negative Bacillary Ventriculitis and Meningitis: A Retrospective Study Evaluating the Efficacy of Intraventricular Gentamicin Therapy in 31 Consecutive Cases, Clin Infect Dis. 2011 Jun;52(11):1310-6.
9. Lesourd A, Magne N, Soares A, et al. Primary bacterial ventriculitis in adults, an emergent diagnosis challenge: report of a meningoccal case and review of the literature. BMC Infect Dis 2018; 18, 226.
10. Hussein K, Bitterman R, Shofty B, et al. Management of post-neurosurgical meningitis: narrative review. Clin Microbiol Infect 2017 Sep;23(9):621-8.
11. Wang JH, Lin PC, Chou CH, et al. Intraventricular antimicrobial therapy in postneurosurgical Gram-negative bacillary meningitis or ventriculitis: a hospital-based retrospective study. J Microbiol Immunol Infect 2014; 47:204–10.
12. Tunkel AR, Hasbun R, Bhimraj A, et al. Infectious Diseases Society of America´s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis 2017 Mar 15;64(6):e34-e65.
13. Hu Y, He W, Yao D, et al. Intrathecal or intraventricular antimicrobial therapy for post-neurosurgical intracranial infection due to multidrug-resistant and extensively drug-resistant Gram-negative bacteria: A systematic review and meta-analysis. Int J Antimicrob Agents 2019 Nov;54(5):556-61.
14. Pai S, Bedford L, Ruramayi R, et al. Pseudomonas aeruginosa meningitis/ventriculitis in a UK tertiary referral hospital, QJM, 2016; 109, pp. 85-9.
15. O’Neill E, Humphreys H, Phillips J, et al. Third-generation cephalosporin resistance among gram[1]negative bacilli causing meningitis in neurosurgical patients: significant challenges in ensuring effective antibiotic therapy. J Antimicrob Chemother 2006; 57:356–9.
16. Shofty B, Neuberger A, Naffaa ME, et al. Intrathecal or intraventricular therapy for post-neurosurgical Gram-negative meningitis: matched cohort study. Clin Microbiol Infect 2016 Jan;22(1):66-70.
17. Blassmann U, Roehr AC, Frey OR, et al. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: a prospective observational study. Crit Care 2016; 20:343.
18. Humphreys H, Jenks P, Wilson J, et al. Surveillance of infection associated with external ventricular drains: proposed methodology and results from a pilot study. J Hosp Infect 2017; 95(2):154–60.



