Introduction
The first description of abnormal artery dilation was by Charles Bougon in 1812.1 Two terms have been used interchangeably to indicate the presence of dilation of coronary vessels: coronary artery aneurysm (CAA) and coronary artery ectasia (CAE), although they actually refer to different phenotypes.2 A focal dilation of a coronary artery exceeding 1.5 times the adjacent normal coronary artery is called CAA, while more diffuse but similar lesions are called CAE.2 CAE is a rare condition with an incidence between 0.2% and 10% on patients undergoing coronary angiograms.3,4One or more of the coronary arteries can be affected, with the right coronary artery being the most commonly involved.3-5 Markis et al published the first prospective study on CAE in 1976 and proposed a classification based on the extent of anatomic involvement:
Type I - diffuse ectasia of two or three vessels;
Type II - diffuse ectasia in one vessel and localized in another;
Type III - diffuse ectasia of one vessel only;
Type IV - localized or segmental ectasia.6
The underlying etiology of CAE is not fully understood and seems to involve both genetic and acquired mechanisms.3,5,7There is no evidence in literature that a single genetic defect can lead to CAE3, but up to 20% of cases are considered congenital.8 Atherosclerosis can be responsible for 50% of cases.8CAE and atherosclerosis share similar cardiovascular risk factors, but the exact link between both conditions has not been well defined.3, 8 It remains unknown why some cases develop ectasia, whereas artery stenosis occurs in the others.3,8 Histological examinations of both diseases show similarities and differences, suggesting that CAE may represent a subtype of atherosclerosis with a distinct pathogenic mechanism.3,8 Recently, Boles et al found an enhanced systemic pro-inflammatory response in CAE with a different cytokine profile from that present in atherosclerosis.8About 10 to 20% of CAE cases are associated with inflammatory or connective tissue diseases such as Ehlers-Danlos syndrome, Kawasaki disease, Marfan’s syndrome, rheumatoid arthritis and Takayasu arteritis.8Iatrogenic aneurysm formation after balloon angioplasty or infections (such as syphilis) are rare causes of CAE.3,4,8
The clinical manifestations of CAE include angina, myocardial infarction, arrhythmia and sudden death.3,5,7 The main recognized mechanism through which CAE leads to myocardial infarction is the slow coronary blood flow observed in ectatic coronaries angiograms, which is thought to promote thrombus formation.3,4,9 CAE-related cytokine patterns may also play a role in this process.3,4,8,9
Case report
We present the case of a 65-year-old man with arterial hypertension, type 2 diabetes mellitus, dyslipidemia, past smoking, previous non-ST elevation myocardial infarction eight years earlier and a recent history of poor adherence to medication. He presented to the emergency department with typical angina that had awoken him. Other than high blood pressure (171/78 mmHg), physical examination at presentation was unremarkable. Initial electrocardiogram showed Q wave in V5, V6 and inferior leads (already present in previous electrocardiograms), a biphasic T wave in leads V3 and V4, flat T wave in V5, DII and aVF and a symmetric inverted T wave in DIII (Fig. 1). High-sensitivity troponin value was 194 ng/L at admission (reference value < 34,2 ng/L), with a maximum value of 37918 ng/L 12 hours later. Echocardiography showed biventricular preserved systolic function with basal inferior localized akinesia. A non-ST elevation myocardial infarction was diagnosed and early coronary angiography was performed revealing: a left coronary artery with diffuse ectasia (Fig. 2); a right dominant coronary artery, with diffuse ectasia and a thrombus on its middle and distal segments, which also involved the posterior descending artery (Fig. 3). No stent was implanted. Dual antiplatelet therapy (with aspirin and ticagrelor) and anticoagulation (with fondaparinux) was started. One week later, repeated coronary angiography showed significant reduction of thrombotic burden. Upon review of medical history, we found that the previous myocardial infarction was also due to a thrombus on the right coronary artery, and both right and left coronaries had already been ectatic. At the time, the patient was also treated with dual antiplatelet therapy (DAPT) and anticoagulation, followed by DATP for a year and then antiplatelet monotherapy with aspirin, which he had recently been taking inconsistently. We decided to prolong triple therapy for one more week and the patient was then discharged under DAPT. After six months of follow-up, no recurrent symptoms were observed.
Discussion
This is a case of type I CAE presenting with recurrent myocardial infarction on the same site of ectasia. As in other cases of myocardial infarction the main goal is to quickly restore coronary blood flow, however, CAE has some particularities that make percutaneous coronary intervention (PCI) a challenging procedure: 1) the size of ectasia can be difficult to delineate due to the thrombus existence; 2) in some cases there is no landing zone to put the stent well apposed to the arterial wall; and 3) there are no specifically designed stents for ectatic coronary arteries.2,4,7 Since CAE is a rare condition, outcome data on PCI in these patients is sparse. Compared to patients without CAE, the procedure has been consistently associated with lower success rate, higher incidence of no-reflow and distal embolization, and also higher rates of subsequent stent thrombosis, restenosis and migration.2,4Routine thrombus aspiration is not recommended, giving the risk of systemic embolization.10It can be an option during PCI in cases of CAE with high thrombus burden.2Intracoronary glycoprotein IIb/IIIa inhibitors or intracoronary thrombolytic may also be considered, especially when the thrombus causes a complete obstruction of the ectatic artery and flow reestablishment is acutely important in patient’s outcome.2The occurrence of no-reflow or distal embolization is also frequent with these strategies.2Surgical interventions have rarely been used in patients not suitable for PCI and in others in whom this procedure failed.2,11,12Surgical approaches are individualized to the coronary anatomy and include ligation or resection of the ectatic artery and bypass grafting across the ectatic segment.2The precise success rate of these techniques is not yet established, as they are rarely used.2
The best long term antithrombotic therapy in patients with CAE is still a matter of debate.2Some studies showed lower rates of myocardial infarction in CAE patients treated with antiplatelet agents compared to those without it, supporting their potential benefit in thrombotic prophylaxis.4Anticoagulation therapy may also be considered, since a recent study from Takahito Doi et al showed a reduction in cardiac death and nonfatal myocardial infarction in patients with CAE treated with warfarin.9In order to define the precise role of each antithrombotic agent or antithrombotic combination in the setting of primary and secondary prevention of myocardial infarction, collaborative studies with clear definitions of the disease phenotypes and clinical endpoints are needed.2While awaiting these studies, treatment strategies should be individualized based on patient characteristics.2
Regarding prognosis, some studies show that CAE patients presenting with myocardial infarction have a higher risk of subsequent major adverse cardiac events than those without ectatic arteries.9,12The risk seems to be higher in type I and type II CAE subtypes and to be influenced by the presence and severity of concomitant coronary artery atherosclerosis.13
Despite our patient having type I CAE, he remained asymptomatic for nearly eight years and myocardial infarction recurrence was related in time to the irregular aspirin intake. As such, we believe the previous strategy with long-term antiplatelet monotherapy to be effective. Based on these observations and on the detection of thrombus resolution after a week of triple antithrombotic therapy, percutaneous and surgical interventions were not considered. Our strategy with triple therapy during the acute phase followed by long-term DAPT has been successful until now. However, this case highlights the need for additional investigation in this area.
Figura I
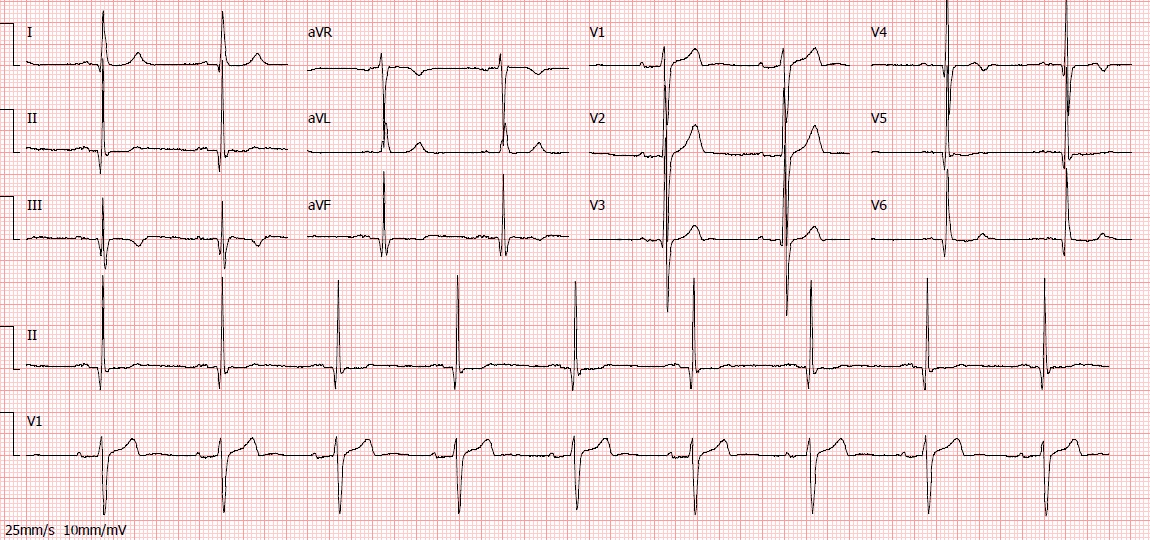
Electrocardiogram at hospital admission.
Figura II
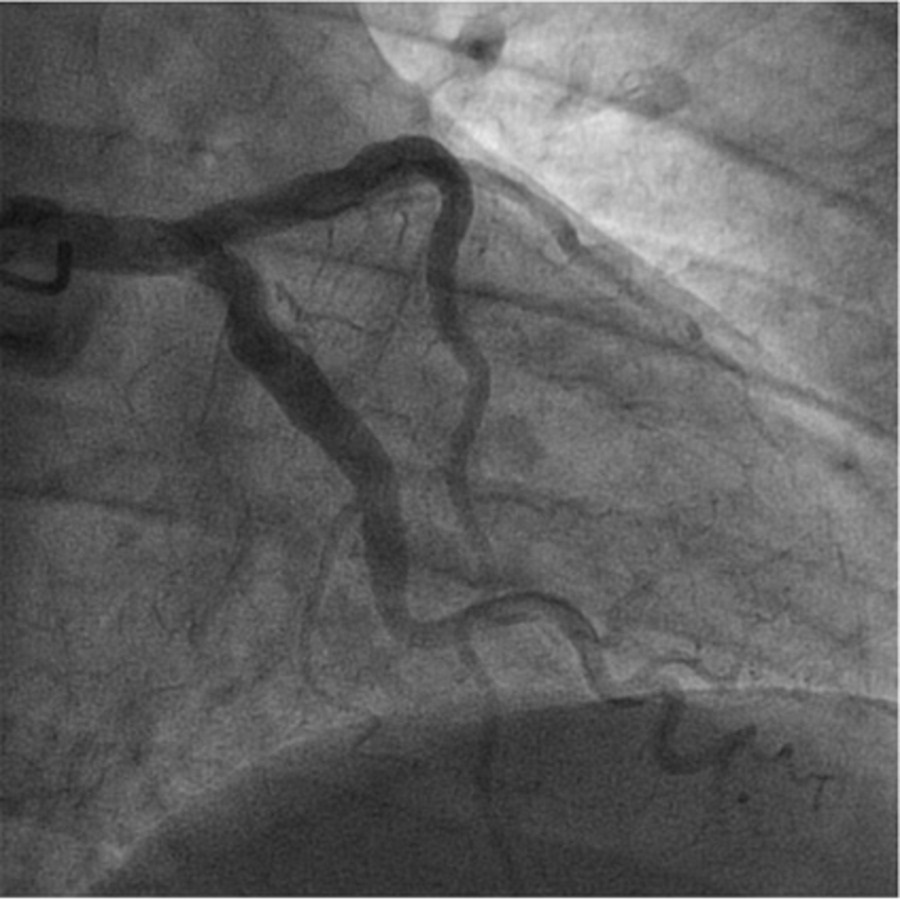
Angiography of the ectatic left coronary artery.
Figura III
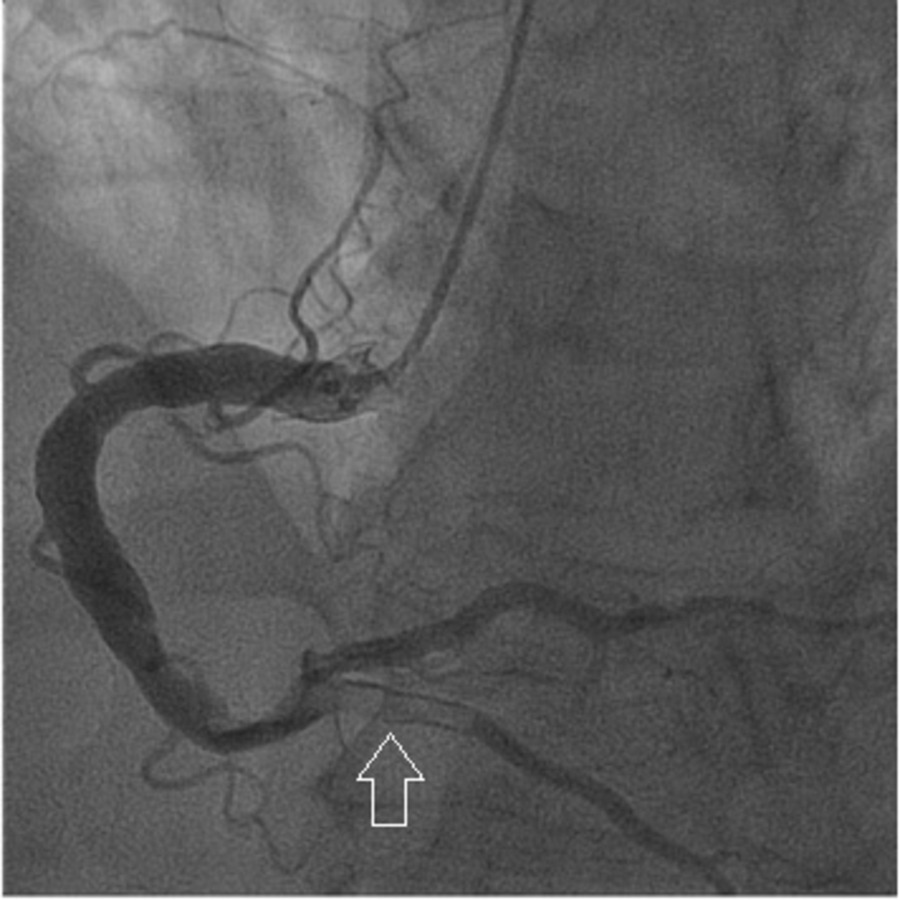
Angiography showing a right dominant coronary artery with diffuse ectasia and a thrombus on the posterior descending artery (arrow).
BIBLIOGRAFIA
1. Jarcho S. Bougon on coronary aneurysm (1812). Am J Cardiol. 1969; 24:551–3. DOI: 10.1016/0002-9149(69)90500-1
2. Kawsara A, Gil IJN, Alqahtani F, Moreland J, Rihal C, Alkhouli M. Management of Coronary Artery Aneurysms. JACC Cardiovasc Interv. 2018; 11:1211-23. DOI: 10.1016/j.jcin.2018.02.041
3. Ozturk S, Yetkin E, Waltenberger J. Molecular and cellular insights into the pathogenesis of coronary artery ectasia. Cardiovasc Path. 2018; 35:37–47. DOI: 10.1016/j.carpath.2018.04.005
4. Liang S, Zhang Y, Gao X, Zhao H, Di B, Sheng Q, et al. Is Coronary Artery Ectasia a Thrombotic Disease? Angiology. 2019;70:62-8. DOI: 10.1177/0003319718782807
5. Latt H, Aung S, Kyaw K, Seher R. Coronary artery ectasia presenting with acute inferior wall myocardial infarction in a young adult. J Community Hosp Intern Med Perspect. 2017; 7:262–4. DOI: 10.1080/20009666.2017.1369376
6. Markis JE, Joffe CD, Cohn PF, Feen DJ, Herman MV, Gorlin R. Clinical significance of coronary arterial ectasia. Am J Cardiol. 1976; 37:217-22. DOI: 10.1016/0002-9149(76)90315-5
7. Chan Y-H, Lam SC-C, Tse H-F. Monozygotic twins with coronary artery ectasia. EurHeartJ CardiovascImaging. 2017; 19:471-2. DOI: 10.1093/ehjci/jex335
8. Boles U, Johansson A, Wiklund U, Wiklund U, Sharif Z, David S, et al. Cytokine Disturbances in Coronary Artery Ectasia Do Not Support Atherosclerosis Pathogenesis. Int J Mol Sci. 2018; 19:260. DOI: 10.3390/ijms19010260
9. Doi T, Kataoka Y, Noguchi T, Shibata T, Nakashima T, Kawakami S, et al. Coronary Artery Ectasia Predicts Future Cardiac Events in Patients With Acute Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2017; 37:2350–5. DOI: 10.1161/ATVBAHA.117.309683
10. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119-77. DOI:10.1093/eurheartj/ehx393
11. Lee J, Ramkumar S, Khav N, Dundon BK. Coronary artery ectasia presenting with ST-elevation myocardial infarction in a young indigenous man: a case report. Eur Heart J Case Rep. 2020;4(5):1-5. DOI:10.1093/ehjcr/ytaa253
12. Bogana Shanmugam V, Psaltis PJ, TL Wong D, T Meredith I, Malaiapan Y et al. Outcomes After Primary Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction Caused by Ectatic Infarct Related Arteries. Heart Lung Circ. 2017;26(10):1059-068. DOI:10.1016/j.hlc.2016.12.006
13.Ahmad M, Mungee S. Coronary Ectasia. StatPearls Publishing (Internet). 2021 (consulted on December 8, 2021). Available on: https://www.ncbi.nlm.nih.gov/books/NBK541130/#_article-20020_s2_




