Introdution:
Dermatomyositis (DM) is a rare autoimmune disease, included in idiopathic inflammatory myositis. Has a singular cutaneous and muscular involvement, but heart, lungs and oesophagus can also be affected. With a prevalence of one to ten cases per million, affects more females in a ratio of 2:1.1 In adults, a peak incidence is between the ages of 40 and 601.
DM is characterized by proximal, progressive and symmetrical muscle weakness associated with pathognomonic skin lesions.1-3 However, symptoms can be non-specific, and severity can vary between patients, making this diagnosis challenging.1,2 Treatment for cutaneous and muscle involvement is required.3
An increased risk of malignancy is described in 15 to 30% of patients.4 Malignancy may precede the appearance of DM or be diagnosed during the follow-up, with a higher risk described in the first year.4,5
This report aims to describe a clinical case of a patient with dermatomyositis and its association with metastatic breast cancer.
Case Report:
A 38-year-old woman, farmer, is hospitalized with progressive asthenia, fatigue, and proximal and symmetrical muscle weakness, involving the scapular and pelvic girdles with four months of evolution. Diffuse muscle pain and limiting functional impact were also mentioned.
The patient reported violaceous maculopapular erythema on her face and back associated with scaly and pruritic erythema on the back of the hands. She denied using new products for hygiene, cleaning or work-related, or new medications.
Physical examination revealed facial oedema and erythema, as well as on the neck and back, with a shawl sign. On the hands, hyperpigmented papular lesions were present, on metacarpophalangeal and proximal interphalangeal joints bilaterally, compatible with Gottron´s papules (Fig. 1). Proximal muscle strength was decreased on upper and lower limbs, with preserved muscle tone and osteotendinous reflexes.
Laboratory studies demonstrated elevation of creatine kinase (CK), with a maximum value of 674 U/L (four times the upper limit of normal). Autoimmunity was positive for myositis, with the presence of anti-TIF-1-γ antibodies. There were no signs of systemic inflammation, with persistently low erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Serological viral markers and infectious serologies were negative.
Complementary studies, with magnetic resonance imaging (MRI) and electromyography, were compatible with inflammatory myositis. Muscle biopsy revealed inflammatory infiltrates, without autophagic vacuoles or amyloid deposits.
Malignancy was excluded through computed tomography (CT) scans of the chest, abdomen and pelvis, endoscopic study, mammography and cervicovaginal cytology. In addition, interstitial lung disease was excluded as well as cardiac or digestive involvement.
Fulfilling the criteria for the diagnosis of dermatomyositis, the patient was prescribed with hydroxychloroquine 200 mg per day, prednisolone 1 mg/kg per day and azathioprine 2.5 mg/kg per day, in association with a physical rehabilitation program.
The patient was discharged after 13 days, with gradual improvement of all symptoms. Minimal residual disease was achieved six months after initial diagnosis, with no functional limitation on daily activities.
Maintaining follow-up on outpatient consultations, no new symptoms or laboratory abnormalities were described until a year after the diagnosis. At that time, the patient experienced a new onset of confusion, jaundice, postprandial infarction, and an increase in abdominal volume. CT scans of the chest, abdomen and pelvis and liver biopsy, revealed metastatic lesions from cancer of an unknown primary site. Mammography and ultrasound uncovered multiple microcalcifications and axillary lymphadenopathy, suggesting metastatic breast cancer. Due to acute liver failure and patient clinical deterioration, breast biopsy was not performed, and taxane-based chemotherapy was started. Despite the treatment, the patient evolved unfavourably and died six days later from progressive disease.
Discussion:
This case report describes a patient with classic characteristics of DM, with pathognomonic violet and infiltrative papular erythema, the Gottron´s papules, accompanied by heliotrope erythema on the upper eyelids. The V-shaped lesions of violaceous photosensitive rash are described on the neckline and back.1,3
Evolving from Bohan and Peter’s criteria, the most recent diagnosis criteria are based on a clinic-serological approach, including specific antibodies, increasing the specificity and sensibility to 88% and 93% respectively.2
Basic laboratory tests include assessment of muscle inflammation, with CK values up to 50 times the upper limit of normal. Although in the presence of an indolent course of the disease, it can be close to normal due to loss of mass muscle.4
Complementary studies with electromyography can provide relevant data suggesting myositis.6 Non-invasive methods as MRI, have high sensitivity to recognize muscle inflammation and are useful to identify the areas where to perform the muscle biopsy, which confers higher accuracy to diagnose.1,3
After the diagnosis, treatment is started to reduce inflammation, minimize symptoms, and improve patients’ quality of life3,6. Physical rehabilitation is required to maintain muscle strength and tone, allowing patients to adapt to daily activities1. Photoprotection should be encouraged to reduce the exacerbating effects of ultraviolet light on the cutaneous disease, and hydroxychloroquine has a recognized benefit.6
To decrease the progression of muscle weakness, glucocorticoids (1mg/kg per day) are the first-line therapy, inducing disease remission in 27-87% of patients.3 Steroid-sparing agents reduces the relapse risk during glucocorticoid tapering and its adverse effects. With better long-term outcomes, azathioprine (2.5-3.0 mg/kg per day) induces remission in about 75% of patients.3
Regarding the association between DM and malignancy, it is described in 15-30% of patients, particularly in males and patients aged 45-74 years at the diagnosis.4,5 Oldroyd et al. reinforced the hypothesis that inflammatory myopathies represent a paraneoplastic syndrome, a reaction immune-mediated in the attempt for clearance of cancer7. Tumour-directed autoantibodies attack autoantigens on muscle and skin cells due to cross-reactivity. And alternatively, treatments with immunosuppressive agents can make patients susceptible to cancer development.7 Malignancy may precede the appearance of DM or be diagnosed during the follow-up, with a higher risk described in the first year.4,5
Exclusion of secondary neoplasms must persist up to three to five years, particularly in the presence of some autoimmune markers.4,5,7,8 Anti- transcription intermediary factor-1 gamma (anti-TIF1-γ) antibody is strongly associated with the presence of malignancy,7 regulating several pathways relevant to cancer development, with a positive predictive value of 84%.8 The more common malignancies associated with DM and promoted by overexpressed anti-TIF1-γ are breast cancer and colorectal cancer.7,8
In this case, despite the regular follow-up, with no abnormal laboratory tests and malignancy being excluded at the time of diagnosis, it emerged as an aggressive metastatic breast cancer within the first year. Although azathioprine may have contributed to increasing the risk of cancer or some hepatotoxicity,6 no elevation of the liver enzymes was documented.
According to current literature, up to 20% of the underlying malignancy associated with DM is breast cancer,4,7 particularly invasive and late-stage tumours.9 When suspected,mammography must be performed and microcalcifications can be the only sign suggesting malignancy on non-palpable breast cancers (described in approximately one-third of the malignant lesions).10 A higher risk is associated with a greater number of microcalcification clusters, but more studies are required.9,10
There are no studies available on the management of breast cancer associated with DM, however some authors support that neoadjuvant chemo or hormonal therapy used for invasive breast cancer can also be applied to these patients.4,11 Combination chemotherapy, like taxane-based schemes, is highly effective for those with extensive or rapidly progressive visceral metastases, in whom the concerns about organ dysfunction are endangering.11 Isolated liver metastases from breast cancer are rare, but the presence is associated with a significant decrease in the survival rate, with a median survival of less than six months.12
Conclusion:
This report intended to illustrate the diagnosis process of DM and the association with underlying malignancy. The challenge on DM diagnosis is based on early recognition, as it may present with general and unspecified symptoms. The pharmacological and non-pharmacological approaches have a prognostic impact, causing major limitations and substantial reduction in quality of life. The coexistence of DM and malignancy is described and cannot be underestimated, even in younger ages with a prior excellent clinical evolution, as it can evolute poorly.
Figura I
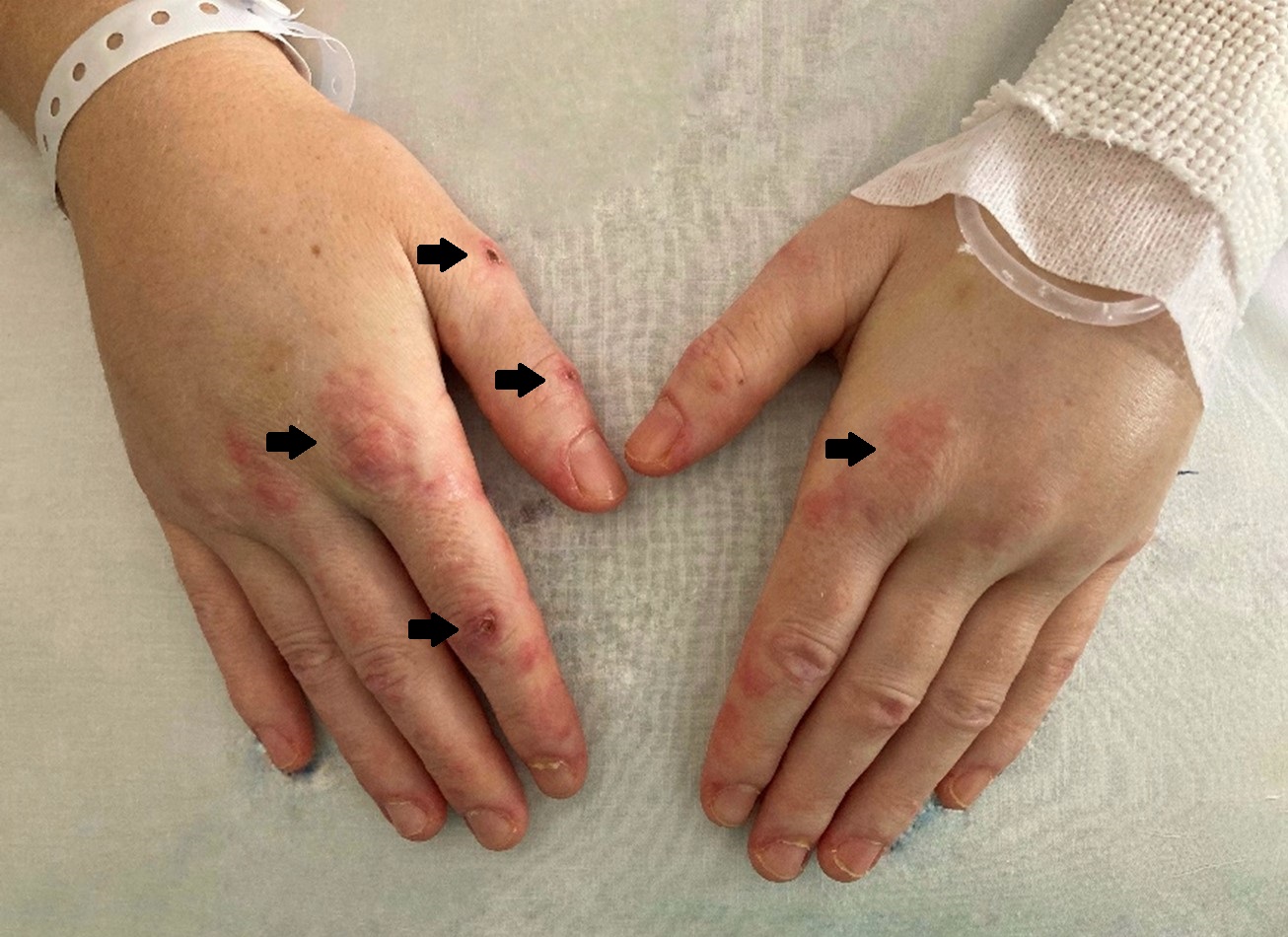
Figure 1: oedema of the hands (stars), with hyperpigmented papular lesions on the metacarpophalangeal and interphalangeal joints (Gottron’ papules) (arrows).
BIBLIOGRAFIA
1-Findlay AR, Goyal NA, Mozaffar T. An overview of polymyositis and dermatomyositis. Muscle and Nerve. 2015, 10.1080/14397595.2018.1467257
2-Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Visser M De, Alfredsson L, et al. EULAR/ACR Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and their Major Subgroups. Ann Rheum Dis. 2017; 76(12): 1955–1964 10.1136/annrheumdis-2017-212709
3-Sasaki H, Kohsaka H. Current diagnosis and treatment of polymyositis and dermatomyositis. Mod Rheumatol. 2018, 10.1080/14397595.2018.1467257
4-Sandhu NP, Zakaria S, Degnim AC, Boughey JC. Dermatomyositis presenting as a paraneoplastic syndrome due to underlying breast cancer. BMJ Case Rep. 2011, 10.1136/bcr.10.2010.3416
5-Bowerman K, Pearson DR, Okawa J, Werth VP. Malignancy in dermatomyositis: A retrospective study of 201 patients seen at the University of Pennsylvania. J Am Acad Dermatol [Internet]. 2020, 10.1016/j.jaad.2020.02.061
6-Lam C, Vleugels RA. Management of cutaneous dermatomyositis. Dermatol Ther. 2012, 10.1111/j.1529-8019.2012.01491.x
7-Oldroyd A, Sergeant JC, New P, McHugh NJ, Betteridge Z, Lamb JA, et al. The temporal relationship between cancer and adult onset anti-transcriptional intermediary factor 1 antibody-positive dermatomyositis. Rheumatology. 2019, 10.1093/rheumatology/key357
8-De Vooght J, Vulsteke JB, De Haes P, Bossuyt X, Lories R, De Langhe E. Anti-TIF1-γ autoantibodies: Warning lights of a tumour autoantigen. Rheumatol (Oxford). 2020; 469-477. 10.1093/rheumatology/kez572
9- Hendren E, Vinik O, Faragalla H, Haq R. Breast cancer and dermatomyositis: A case study and literature review. Curr Oncol. 2017; 24(5):e429–33.
10- Azam S, Eriksson M, Sjölander A, Gabrielson M, Hellgren R, Czene K, et al. Mammographic microcalcifications and risk of breast cancer. Br J Cancer. 2021;125(5):759–65.
11-Lee JH, Nan A. Combination Drug Delivery Approaches in Metastatic Breast Cancer. J Drug Deliv. 2012, 10.1155/2012/915375
12- Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: Long-term survival after curative resection. Surgery. 2000;127(4):383–9.


